Table of Contents - Issue
Recent articles
-
 A Comprehensive Review on Therapeutic Implications of Medicinal Plants in Ovarian CancerAuthor: Ponnulakshmi RDOI: 10.21522/TIJPH.2013.SE.25.01.Art001
A Comprehensive Review on Therapeutic Implications of Medicinal Plants in Ovarian CancerAuthor: Ponnulakshmi RDOI: 10.21522/TIJPH.2013.SE.25.01.Art001A Comprehensive Review on Therapeutic Implications of Medicinal Plants in Ovarian Cancer
Abstract:
Although ovarian cancer is the fifth most common cancer in women, it is the eleventh most common cancer. Ovarian cancer accounts for about 2.5% of all cancers in women. The most lethal type of gynecologic cancer is ovarian cancer. Ovarian cancer treatment has become more challenging as a result of inadequate early diagnosis and chemoresistance. Every microanatomic subtype had a specific molecular and epigenetic fingerprint associated with it. According to its histology, OC was divided into four subtypes, of which epithelial ovarian cancer (EOC) is more common than the others. Medicinal plants have contributed a vital role in the therapy of cancer. since ancient times, and the advantage of herbs is that they are less toxic to the human system compared to commercially available drugs. The majority of medicinal plants increase the effectiveness of chemotherapy, allowing us to reduce patient chemoresistance, which caught the attention of researchers. In this overview, we emphasize the role of Scutellaria barbata, Camellia sinensis, curcumin, ashwagandha, Leea indica, Garcinia, Asparagus and Cnidium monnier in ovarian cancer cells' molecular mechanisms.
A Comprehensive Review on Therapeutic Implications of Medicinal Plants in Ovarian Cancer
References:
[1]. Kurman, R. J., & Shih, I. M., 2011, Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer–shifting the paradigm. Hum. Pathol. 42, 918–931. 10.1016/j.humpath.2011.03.003.
[2]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P. & Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), pp.1007-1013.
[3]. Krishnan, R. P., Pandiar, D., Ramani, P. and Jayaraman, S., 2025, Molecular profiling of oral epithelial dysplasia and oral squamous cell carcinoma using next generation sequencing. Journal of Stomatology, Oral and Maxillofacial Surgery, 126(4), p.102120.
[4]. Tavassoli, M., Ruhrberg, C., Beaumont, V., Reynolds, K., Kirkham, N., Collins, W. P., & Farrin Farzaneh, F., 1993, Whole Chromosome 17 Loss in Ovarian Cancer. GENES, CHROMOSOMES & CANCER &I95498. 10.1002/gcc.2870080310.
[5]. Chisholm, K. M., Goff, B. A., Garcia, R., King, M. C. & Swisher, E. M., 2008, Genomic structure of chromosome 17 deletions in BRCA1 associated ovarian cancers. Cancer Genet Cytogenet. May; 183(1): 41–48. 10.1016/j.cancergencyto.2008.02.00.
[6]. Shen H, Fridley B. L, Song H, Lawrenson K, Cunningham J. M, Ramus S. J, Cicek M. S, Tyrer J, Stram D, Larson M. C, 2013, Epigenetic analysis leads to identification of HNF1B as a subtype-specific susceptibility gene for ovarian cancer. Nat. Commun 2013, 4. 10.1038/ncomms2629.
[7]. Mabuchi, S., Kawase, C., Altomare, D. A., Morishige, K., Sawada, K., Hayashi, M., Tsujimoto, M., Yamoto, M., Klein-Szanto, A. J., Schilder, R. J., Ohmichi, M., Testa, J. R., & Kimura, T., 2009, mtor is a promising therapeutic target both in cisplatin-sensitive and cisplatin-resistant clear cell carcinoma of the ovary. Clin Cancer Res., 15(17), 5404–5413. doi: 10.1158/1078-0432.CCR-09-0365.
[8]. Jayson, G. C., Kohn, E. C., Kitchener, H. C., & Ledermann, J. A., 2014, Ovarian Cancer. The Lancet, 384, 1376–1388. 10.1016/S0140-6736(13)62146-7.
[9]. Banerjee, S., & Kaye, S. B., 2013, New strategies in the treatment of ovarian cancer: Current clinical perspectives and future potential. Clinical Cancer Research, 19, 961–968. 10.1158/1078-0432.CCR-12-2243.
[10]. Sruthi, M. A., Mani, G., Ramakrishnan, M. and Selvaraj, J., 2023, Dental caries as a source of Helicobacter pylori infection in children: An RT‐PCR study. International Journal of Paediatric Dentistry, 33(1), pp.82-88.
[11]. Chen, Q., Rahman, K., Wang, S.-J., Zhou, S., & Zhang, H., 2019, Scutellaria barbata: A Review on Chemical Constituents, Pharmacological Activities and Clinical Applications. PMID: 31840605. 10.2174/1381612825666191216124310.
[12]. Gao, J., Yin, W., & Corcoran, O., 2019, From Scutellaria barbata to BZL101 in Cancer Patients: Phytochemistry, Pharmacology, and Clinical Evidence. Journal of Natural Medicines. 10.1177/1934578X19880645.
[13]. Lin, Z., Ren, B., Zhang, J., Liu, L., Liu, J., Jiang, G., Li, M., Ding, Y., & Li, W., 2017, Anti-tumor effect of Scutellaria barbata D. Don extracts on ovarian cancer and its phytochemicals characterisation. Journal of Ethnopharmacology, 206, 184-192. 10.1016/j.jep.2017.05.032.
[14]. Xiao, X., Chen, F., Zhang, L., Liu, L., Zhang, C., Zhang, Z., & Li, W., 2021, Exploring the mechanisms of anti-ovarian cancer of Hedyotis diffusa Willd and Scutellaria barbata D. Don through focal adhesion pathway. Journal of Ethnopharmacology. 10.1016/j.jep.2021.114343.
[15]. Jie, L., Wang, Y., Lei, J.-C., Hao, Y., Yang, Y., Yang, C.-X., & Yu, J.-Q., 2014, Sensitisation of ovarian cancer cells to cisplatin by flavonoids from Scutellaria barbata. Natural Product Research, 28(10), 683–689. 10.1080/14786419.2013.871547.
[16]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P. and Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), pp.704-713.
[17]. Wang, Z.-L., Wang, S., Kuang, Y., Hu, Z.-M., Qiao, X., & Ye, M., 2018, A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharmaceutical Biology, 56(1), 465–484. 10.1080/13880209.2018.1492620.
[18]. Wogonin | C16H12O5 | CID 5281703 – PubChem.
[19]. Jiang, R., Jin, B., Wan, D., Zhu, C., Feng, J., & Gu, L., 2017, Therapy Effects of Wogonin on Ovarian Cancer Cells. BioMed Research International, 1–8. 10.1155/2017/9381513.
[20]. Feng, X., Cong, S., Ning, L., Yuanying, H., Mengmeng, C., Siyu, D., Chenghua, L., Lijin, F., & Zhongping, C., 2019, Wogonin Increases Cisplatin Sensitivity in Ovarian Cancer Cells Through Inhibition of the Phosphatidylinositol 3-Kinase (PI3K)/Akt Pathway. Med Sci Monit., 25, 6007–6014. 10.12659/MSM.913829.
[21].Nemecz, G., 2002, Green tea. The Journal of Modern Pharmacy.
[22]. Roychoudhury, S., Halenar, M., Michalcova, K., Natha, S., Kacaniova, M., & Kolesarova, A., 2018, Green tea extract affects porcine ovarian cell apoptosis. Reprod Biol., 18(1), 94–98. 10.1016/j.repbio.2018.01.007.
[23]. Spinella, F., Rosano, L., Di Castro, V., Decandia, S., Albini, A., Nicotra, M. R., Natali, P. G., & Bagnato, A., 2006, Green tea polyphenol epigallocatechin-3-gallate inhibits the endothelin axis and downstream signaling pathways in ovarian carcinoma. Mol Cancer Ther., 5(6), 1483–1492. 10.1158/1535-7163.MCT-06-0053.
[24]. Huh, S. W., Bae, S. M., Han, C. H., Choi, J. H., Kim, C. K., Park, E. K., Ro, D. Y., Lee, J. M., Namkoong, S. E., & Ahn, W. S., 2004, Anti-Tumor Effects of Epigallocatechin-3-Gallate Extracted From Green Tea On Ovarian Cancer Cell Lines. Korean Journal of Obstetrics and Gynecology, 634-649.
[25]. Yasothkumar, D., Ramani, P., Jayaraman, S., Ramalingam, K. and Tilakaratne, W. M., 2024, Expression Profile of Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck Pathology, 18(1), p.28.
[26]. Pianetti, S., Guo, S., Kavanagh, K. T., & Sonenshein, G. E., 2002, Green tea polyphenol epigallocatechin-3 gallate inhibits Her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Research, 62(3), 652-655.
[27]. Przystupski, D., Michel, O., Rossowska, J., Kwiatkowski, S., Saczko, J., & Kulbacka, J., 2019, The modulatory effect of green tea catechin on drug resistance in human ovarian cancer cells. Medicinal Chemistry Research, 28, 657-667.
[28]. Sicard, A. A., Gonzalez Suarez, N., Cappadocia, L., & Annabi, B., 2021,Functional targeting of the TGF-βR1 kinase domain and downstream signaling: A role for the galloyl moiety of green tea-derived catechins in ES-2 ovarian clear cell carcinoma. The Journal of Nutritional Biochemistry, 87, 108518. https://doi.org/10.1016/j.jnutbio.2020.108518
[29]. Maity, R., Chatterjee, M., Banerjee, A., Das, A., Mishra, R., Mazumder, S., & Chanda, N., 2019, Gold nanoparticle-assisted enhancement in the anti-cancer properties of theaflavin against human ovarian cancer cells. Materials Science and Engineering: C, 109909. https://doi.org/10.1016/j.msec.2019.109909
[30]. Zhang, M., Lee, A. H., Binns, C. W., & Xie, X., 2004, Green tea consumption enhances survival of epithelial ovarian cancer. International Journal of Cancer, 112(3), 465–469. https://doi.org/10.1002/ijc.20456
[31]. Pazhani, J., Chanthu, K., Jayaraman, S. and Varun, B. R., 2023, Evaluation of salivary MMP-9 in oral squamous cell carcinoma and oral leukoplakia using ELISA. Journal of Oral and Maxillofacial Pathology, 27(4), pp.649-654.
[32]. Panji, M., Behmard, V., Zare, Z., Malekpour, M., Nejadbiglari, H., Yavari, S., Nayerpoudizaj, T., Safaeian, A., Bakhshi, A., Abazari, O., Abbasi, M., Khanicheragh, P., & Shabanzadeh, M., 2021, Synergistic effects of green tea extract and paclitaxel in the induction of mitochondrial apoptosis in ovarian cancer cell lines. Gene, 787, 145638. 10.1016/j.gene.2021.145638.
[33]. Fathima, J. S., Jayaraman, S., Sekar, R. and Syed, N. H., 2024, The role of MicroRNAs in the diagnosis and treatment of oral premalignant disorders. Odontology, pp.1-10.
[34]. Chen, H., Landen, C. N., Li, Y., Alvarez, R. D., & Tollefsbol, T. O., 2013, Epigallocatechin gallate and sulforaphane combination treatment induce apoptosis in paclitaxel-resistant ovarian cancer cells through hTERT and Bcl-2 down-regulation. Experimental Cell Research, 319(5), 697–706. http://dx.doi.org/10.1016/j.yexcr.2012.12.026.
[35]. Lestari, M. L. A. D., & Indrayanto, G., 2015, Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Curcumin. 10.1016/b978-0-12-800173-8.00003-9. doi: 10.1016/j.cellbi.2005.10.024
[36]. Shishodia, S., Chaturvedi, M. M., & Aggarwal, B. B., 2007, Role of curcumin in cancer therapy. Current Problems in Cancer, 31, 243-305. 10.1016/j.currproblcancer.2007.04.001
[37]. Sahin, K., Orhan, C., Tuzcu, M., Sahin, N., Tastan, H., Özercan, İ. H., Güler, O., Kahraman, N., Kucuk, O., & Ozpolat, B., 2017, Chemopreventive and antitumor efficacy of curcumin in a spontaneously developing hen ovarian cancer model. Cancer Prevention Research, PMID: 29089332. DOI: 10.1158/1940-6207.CAPR-16-0289
[38]. Murali, M. Yallapu, Diane M. Maher, Vasudha Sundram, Maria C. Bell, Meena Jaggi, & Subhash C. Chauhan, 2010, Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. Journal of Ovarian Research, 3, Article 11. 10.1186/1757-2215-3-11.
[39]. Sagar, S., Ramani, P., Moses, S., Gheena, S. and Selvaraj, J., 2024, Correlation of salivary cytokine IL-17A and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology, pp.1-10.
[40]. Wei, P., Hui, Y., Cong, C., Xiuzu, S., Brittany, W., Rebecca, K., Shan, L., Gang, H., Wen, D., & Yinsheng, W., 1994, AMPK mediates curcumin-induced cell death in CAOV3 ovarian cancer cells. Oncology Reports. 10.3892/or_00000179.
[41]. Seo, J., Kim, B., Dhanasekaran, D. N., Tsang, B. K., & Song, Y. S., 2016, Curcumin induces apoptosis by inhibiting sarco/endoplasmic reticulum Ca2+ ATPase activity in ovarian cancer cells. Cancer Letters, 371(1), 30-37. 10.1016/j.canlet.2015.11.021.
[42]. Yuzhu, H., Mengni, R., Bilan, W., Yunzhu, L., Yongzhong, C., & Songping, Z., 2020, Co-Delivery of Docetaxel and Curcumin via Nanomicelles for Enhancing Anti-Ovarian Cancer Treatment. International Journal of Nanomedicine, 15, 9703-9715. 10.2147/IJN.S274083.
[43]. Muhanmode, Y., Yalikun, W., Meng Ke, M., Maitinuri, A., Amina, S., & Shen, G., 2022, Curcumin and resveratrol inhibit chemoresistance in cisplatin-resistant epithelial ovarian cancer cells via targeting PI3K pathway. Human & Experimental Toxicology, 41, 9603271221095929. 10.1177/09603271221095929.
[44]. Mishra, L. C., Singh, B. B., & Dagenais, S., 2000, Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): A review. Alternative Medicine Reviews, 5, 334-346.
[45]. Bhattacharya, S. K., Goel, R. K., Kaur, R., & Ghosal, S., 1987, Anti-stress activity of Sitoindosides VII and VIII. New Acylsterylglucosides from Withania somnifera. Phytotherapy Research, 1, 32-37.
[46]. Barua, A., Bradaric, M. J., Bitterman, P., Abramowicz, J. S., Sharma, S., Basu, S., Lopez, H., Bahr, J. M., 2013, Dietary Supplementation of Ashwagandha (Withania somnifera, Dunal) Enhances NK Cell Function in Ovarian Tumors in the Laying Hen Model of Spontaneous Ovarian Cancer. AJI, 10.1111/aji.12172.
[47]. Kakar, S. S., Ratajczak, M. Z., Powell, K. S., Moghadamfalahi, M., Miller, D. M., Batra, S. K., & Singh, S. K., 2014, Withaferin A Alone and in Combination with Cisplatin Suppresses Growth and Metastasis of Ovarian Cancer by Targeting Putative Cancer Stem Cells. PLOS ONE, 10.1371/journal.pone.0107596.
[48]. Neo, S. Y., Siew, Y. Y., Yew, H. C., He, Y., Poh, K. L., Tsai, Y. C., Ng, S. L., Tan, W. X., Chong, T. I., Lim, C. S. E. S., Ho, S. W., Singh, D., Ali, A., Linn, Y. C., Tan, C. H., Seow, S. V., & Koh, H. L., 2023, Effects of Leea indica leaf extracts and its phytoconstituents on natural killer cell-mediated cytotoxicity in human ovarian cancer. BMC Complementary Medicine and Therapies, 10.1186/s12906-023-03904-1.
[49]. Sairam, K., Priyambada, S., Aryya, N. C., & Goel, R. K., 2003, Gastroduodenal ulcer protective activity of Asparagus racemosus: an experimental, biochemical and histological study. Biological and Pharmaceutical Bulletin, PMID: 12686434, 10.1016/s0378-8741(02)00342-2.
[50]. Kalai Vani, S., Sivakumar, G., Geetha, R. V., & Vishnu Priya, V., 2019, Invitro cytotoxic activity of Asparagus racemosus on ovarian carcinoma cell lines (SKOV-3) by 3-(4, 5-dimethythiazol-2-yl) -2, 5-diphenyl tetrazolium bromide assay. Drug Invention Today, 12(6), 1162.
[51]. Zhang, X., Wang, J., Fan, Y., Zhao, Z., Paraghamian, S. E., Hawkins, G. M., Buckingham, L., O’Donnell, J., Hao, T., Suo, H., Yin, Y., Sun, W., Kong, W., Sun, D., Zhao, L., Zhou, C., & Bae Jump, V. L., 2023, Asparagus officinalis combined with paclitaxel exhibited synergistic anti-tumor activity in paclitaxel sensitive and resistant ovarian cancer cells. Journal of Cancer Research and Clinical Oncology, 149, 3871–3883.
[52]. Hemashekhar, M., Sunitha, K., Santhosh, M. S., Devaraja, S., Kemparaju, K., Vishwanath, B. S., Niranjana, S. R., & Girish, K. S., 2011, An overview on genus Garcinia: Phytochemical and therapeutical aspects. Phytochemistry Reviews, 10, 325-351. https://doi.org/10.1007/s11101-011-9207-3
[53]. Yang, R., Li, P., Li, N., Zhang, Q., Bai, X., Wang, L., Xiao, Y., Sun, L., Yang, Q., & Yan, J., 2017, Xanthones from the pericarp of Garcinia mangostana. Molecules, 22(5), 683. https://doi.org/10.3390/molecules22050683.
[54]. https://pubchem.ncbi.nlm.nih.gov/compound/10298511.
[55]. Xu, X. H., Liu, Q. Y., Li, T., Liu, J. L., Chen, X., Huang, L., Qiang, W. A., Chen, X., Wang, Y., Lin, L. G., & Lu, J. J., 2017, Garcinone E induces apoptosis and inhibits migration and invasion in ovarian cancer cells. Scientific Reports, 7, 10718. https://doi.org/10.1038/s41598-017-11417-4
[56]. https://pubchem.ncbi.nlm.nih.gov/substance/134222836.
[57]. Setyawati, L. U., Nurhidayah, W., Khairul Ikram, N. K., Mohd Fuad, W. E., & Muchtaridi, M., 2023, General toxicity studies of alpha mangostin from Garcinia mangostana: A systematic review. Heliyon, 9(5):e16045. doi:10.1016/j.heliyon.2023.e16045.
[58]. Ittiudomrak, T., Puthong, S., Roytrakul, S., & Chanchao, C., 2019, α-Mangostin and Apigenin Induced Cell Cycle Arrest and Programmed Cell Death in SKOV-3 Ovarian Cancer Cells. Toxicological Research, 35(2), 167–179. doi:10.5487/TR.2019.35.2.167.
[59]. Yu, Y., Fei, Z., & Qin, L., 2020, Anticancer effects of α-mangostin in OVACAR-3 human ovarian carcinoma cells are mediated via involvement of reactive oxygen species, mitochondrial –mediated apoptosis, suppression of cell migration and invasion and m-TOR/PI3K/AKT signaling pathway. JBUON, 25(5), 2294.
[60]. Zhang, Z.-R., Leung, W. N., Cheung, H. Y., & Chan, C. W., 2015, Osthole: A Review on Its Bioactivities, Pharmacological Properties, and Potential as Alternative Medicine. Evidence-Based Complementary and Alternative Medicine, 2015, 1–10. doi:10.1155/2015/919616.
[61]. Jiang, G., Liu, J., Ren, B., Tang, Y., Owusu, L., Li, M., Zhang, J., Liu, L., & Li, W., 2016, Anti-tumor effects of osthole on ovarian cancer cells in vitro. Journal of Ethnopharmacology, 193, 368–376. doi:10.1016/j.jep.2016.08.045.
[62]. Liang, J., Zhou, J., Xu, Y., Huang, X., Wang, X., Huang, W., & Li, H., 2020, Osthole inhibits ovarian carcinoma cells through LC3-mediated autophagy and GSDME-dependent pyroptosis except for apoptosis. European Journal of Pharmacology, 874, 172990. doi:10.1016/j.ejphar.2020.172990.
Viewed PDF 356 69 -
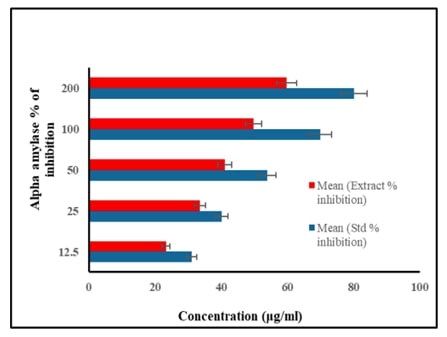 Effect of Natural Flavonoid Apigenin in Lowering High Glucose-Induced Insulin Resistance via Targeting PI3K/AKT Pathway in 3T3-L1 Adipocytes – Evidence Through an In-vitro and In-silico ApproachAuthor: Selvaraj JayaramanDOI: 10.21522/TIJPH.2013.SE.25.01.Art002
Effect of Natural Flavonoid Apigenin in Lowering High Glucose-Induced Insulin Resistance via Targeting PI3K/AKT Pathway in 3T3-L1 Adipocytes – Evidence Through an In-vitro and In-silico ApproachAuthor: Selvaraj JayaramanDOI: 10.21522/TIJPH.2013.SE.25.01.Art002Effect of Natural Flavonoid Apigenin in Lowering High Glucose-Induced Insulin Resistance via Targeting PI3K/AKT Pathway in 3T3-L1 Adipocytes – Evidence Through an In-vitro and In-silico Approach
Abstract:
Diabetes mellitus, characterized by elevated blood glucose levels resulting from insulin deficiency or resistance, poses a significant global health challenge. With its increasing prevalence and substantial impact on morbidity, mortality, and healthcare costs, effective strategies for managing diabetes are urgently needed. Natural flavonoid such as apigenin, has emerged as potential therapeutic agent due to their antioxidant, anti-inflammatory anti-diabetic properties but mechanism of action is not known. The study was aimed at assessing the role of apigenin on PI3K/AKT/GLUT4 pathway in 3T3-L1 adipocytes. Invitro alpha amylase and alpha glucosidase inhibitory activity was measured by spectrophotometric methods. Cytotoxicity was assessed by MTT assay. Further, gene expression analysis was done by Real Time-PCR. In order to confirm the exact binding interaction of apigenin with PI3K/Akt/GLUT4 signaling, molecular docking analysis was also performed. Results of this study showed that apigenin significantly reduced alpha amylase and alpha glucosidase inhibitory activity in a dose-dependent fashion. q-PCR analysis showed that apigenin significantly improved mRNA expression of insulin signaling molecules (IR, IRS-1, PI3K, Akt and GLUT4) in high glucose-induced 3T3-L1 adipocytes cell line. Molecular docking analysis evidenced that apigenin confirmed possible role of apigenin that regulates insulin metabolic signaling in adipocytes. Overall, apigenin holds promise as a natural flavonoid with potential therapeutic value in combating diabetes and its complications, underscoring the importance of continued research to unlock its full therapeutic potential and pave the way for effective diabetes management strategies.
Effect of Natural Flavonoid Apigenin in Lowering High Glucose-Induced Insulin Resistance via Targeting PI3K/AKT Pathway in 3T3-L1 Adipocytes – Evidence Through an In-vitro and In-silico Approach
References:
[1]. Salehi, B., Venditti, A., Sharifi-Rad, M., Kręgiel, D., Sharifi-Rad, J., Durazzo, A., Lucarini, M., Santini, A., Souto, E. B., Novellino, E., Antolak, H., Azzini, E., Setzer, W. N., & Martins, N., 2019, The Therapeutic Potential of Apigenin. International Journal of Molecular Sciences, 20(6), 1305. https://doi.org/10.3390/ijms20061305
[2]. Selvaraj, J., Veeraraghavan, V., Periyasamy, V., and Rajagopal, P., 2021., In Silico and in Vitro Study on the Inhibition of FtsZ Protein of Staphylococcus Aureus by Active Compounds from Andrographis Paniculata. Journal of Biologically Active Products from Nature, 11(2): 116–128. doi:10.1080/22311866.2021.1908163.
[3]. Ponnulakshmi, R., Shyamaladevi, B., Vijayalakshmi, P., & Selvaraj, J., 2019, In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicology Mechanisms and Methods, 29(4): 276–290. https://doi.org/10.1080/15376516.2018.1545815.
[4]. Babu, S., Jayaraman, S., 2020, An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed Pharmacother, 131:110702. doi: 10.1016/j.biopha.2020.110702. Epub 2020 Aug 31. PMID: 32882583.
[5]. Jayaraman, S., Roy, A., Vengadassalapathy, S., Sekar, R., Veeraraghavan, V. P., Rajagopal, P., Rengasamy, G., Mukherjee, R., Sekar, D., Manjunathan, R., 2021, An Overview on the Therapeutic Function of Foods Enriched with Plant Sterols in Diabetes Management. Antioxidants.; 10(12):1903. https://doi.org/10.3390/antiox10121903
[6]. Jayaraman, S., Devarajan, N., Rajagopal, P., Babu, S., Ganesan, S. K., Veeraraghavan, V. P., Palanisamy, C. P., Cui, B., Periyasamy, V., Chandrasekar, K., 2021, β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules; 26(7):2101. https://doi.org/10.3390/molecules26072101.
[7]. Pei, J., Yan, Y., Jayaraman, S., Rajagopal, P., Natarajan, P. M., Umapathy, V. R., Gopathy, S., Roy, J. R., Sadagopan, J. C., Thalamati, D., Palanisamy, C. P., Mironescu, M., 2024, A review on advancements in the application of starch-based nanomaterials in biomedicine: Precision drug delivery and cancer therapy. Int J Biol Macromol, 265(Pt 1):130746. doi: 10.1016/j.ijbiomac.2024.130746. Epub ahead of print. PMID: 38467219.
[8]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P., & Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8): 1007-1013.
[9]. Hatano, T., Edamatsu, R., Hiramatsu, M., MORI, A., Fujita, Y., Yasuhara, T., & OKUDA, T., 1989, Effects of the interaction of tannins with co-existing substances. VI.: effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-Diphenyl-2-picrylhydrazyl radical. Chemical and pharmaceutical bulletin, 37(8), 2016-2021.
Viewed PDF 251 25 -
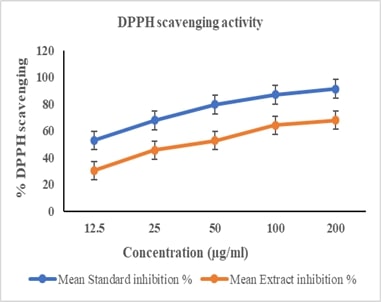 Molecular Mechanisms Underlying in the Anticancer Activity of Verbacoside Against Human Lung Adeno Carcinoma (A549) Cells Via Modulating Apoptotic SignallingAuthor: Selvaraj JayaramanDOI: 10.21522/TIJPH.2013.SE.25.01.Art003
Molecular Mechanisms Underlying in the Anticancer Activity of Verbacoside Against Human Lung Adeno Carcinoma (A549) Cells Via Modulating Apoptotic SignallingAuthor: Selvaraj JayaramanDOI: 10.21522/TIJPH.2013.SE.25.01.Art003Molecular Mechanisms Underlying in the Anticancer Activity of Verbacoside Against Human Lung Adeno Carcinoma (A549) Cells Via Modulating Apoptotic Signalling
Abstract:
Verbascoside (VERB), a phenylethanoid-phenylpropanoid glycoside, has garnered significant interest due to its potential therapeutic effects, particularly its anticancer properties. This study investigates the molecular mechanisms underlying the anticancer activity of VERB against A549 cells, a model of non-small cell lung cancer (NSCLC). Our findings demonstrate that VERB induces apoptosis in A549 cells through the modulation of key apoptotic signaling pathways. Specifically, VERB treatment resulted in the activation of caspases, upregulation of pro-apoptotic proteins, and downregulation of anti-apoptotic proteins. Additionally, VERB was observed to inhibit the NF-kB pathway, thereby reducing inflammation and promoting apoptotic cell death. These results suggest that VERB exerts its anticancer effects by targeting multiple cellular pathways involved in cell survival and apoptosis, providing a promising avenue for the development of novel NSCLC therapies.
Molecular Mechanisms Underlying in the Anticancer Activity of Verbacoside Against Human Lung Adeno Carcinoma (A549) Cells Via Modulating Apoptotic Signalling
References:
[1]. Siegel, R. L., Miller, K. D., & Jemal, A., 2018, Cancer statistics, 2018. CA: a cancer journal for clinicians, 68(1), 7-30.
[2]. Ames, B. N., & Gold, L. S., 1998, The causes and prevention of cancer: the role of environment. Biotherapy, 11, 205-220.
[3]. Yeung, S. C., Habra, M. A., & Thosani, S. N., 2011, Lung cancer-induced paraneoplastic syndromes. Current opinion in pulmonary medicine, 17(4), 260–268. https://doi.org/10.1097/MCP.0b013e328347bdba.
[4]. Van Schil, P. E., Sihoe, A. D., & Travis, W. D., 2013, Pathologic classification of adenocarcinoma of lung. Journal of Surgical Oncology, 108(5), 320–326. https://doi.org/10.1002/jso.23397.
[5]. Li, X., Wang, S., Zhu, R., Li, H., Han, Q., & Zhao, R. C. , 2016, Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFκB-TLR signaling pathway. Journal of Hematology & Oncology, 9, 42. https://doi.org/10.1186/s13045-016-0269-y.
[6]. Al-Harbi, N. O., Imam, F., Al-Harbi, M. M., Ansari, M. A., Zoheir, K. M., Korashy, H. M., Sayed-Ahmed, M. M., Attia, S. M., Shabanah, O. A., & Ahmad, S. F., 2016, Dexamethasone Attenuates LPS-induced Acute Lung Injury through Inhibition of NF-κB, COX-2, and Pro-inflammatory Mediators. Immunological Investigations, 45(4), 349–369. https://doi.org/10.3109/08820139.2016.1157814.
[7]. Lu, W. J., Lin, K. H., Hsu, M. J., Chou, D. S., Hsiao, G., & Sheu, J. R., 2012, Suppression of NF-κB signalling by andrographolide with a novel mechanism in human platelets: regulatory roles of the p38 MAPK-hydroxyl radical-ERK2 cascade. Biochemical Pharmacology, 84(7), 914–924. https://doi.org/10.1016/j.bcp.2012.06.030
[8]. Desai, B. N., Myers, B. R., & Schreiber, S. L., 2002, FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proceedings of the National Academy of Sciences of the United States of America, 99(7), 4319–4324. https://doi.org/10.1073/pnas.261702698.
[9]. Luo, C., Zhu, Y., Jiang, T., Lu, X., Zhang, W., Jing, Q., Li, J., Pang, L., Chen, K., Qiu, F., Yu, X., Yang, J., & Huang, J., 2007, Matrine induced gastric cancer MKN45 cells apoptosis via increasing pro-apoptotic molecules of Bcl-2 family. Toxicology, 229(3), 245–252. https://doi.org/10.1016/j.tox.2006.10.020.
[10]. Liang, C. Z., Zhang, J. K., Shi, Z., Liu, B., Shen, C. Q., & Tao, H. M., 2012, Matrine induces caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemotherapy and Pharmacology, 69(2), 317–331. https://doi.org/10.1007/s00280-011-1699-4
[11]. Nussbaumer, S., Bonnabry, P., Veuthey, J. L., & Fleury-Souverain, S., 2011, Analysis of anticancer drugs: a review. Talanta, 85(5), 2265–2289. https://doi.org/10.1016/j.talanta.2011.08.034
[12]. Rostamabadi, H., Falsafi, S. R., & Jafari, S. M., 2019, Nanoencapsulation of carotenoids within lipid-based nanocarriers. Journal of Controlled Release: Official Journal of the Controlled Release Society, 298, 38–67. https://doi.org/10.1016/j.jconrel.2019.02.005.
[13]. Gil, E. deS., Enache, T. A., & Oliveira-Brett, A. M., 2013, Redox behaviour of verbascoside and rosmarinic acid. Combinatorial Chemistry & High Throughput Screening, 16(2), 92–97.
[14]. Oyourou, J. N., Combrinck, S., Regnier, T., & Marston, A., 2013, Purification, Stability and Antifungal Activity of Verbascoside from Lippia javanica and Lantana camara Leaf Extracts. Industrial Crops and Products, 43, 820-826. https://doi.org/10.1016/j.indcrop.2012.08.028.
[15]. Fan, Y., Xu, C., Li, J., Zhang, L., Yang, L., Zhou, Z., Zhu, Y., & Zhao, D., 2018, Ionic liquid-based microwave-assisted extraction of verbascoside from Rehmannia root. Industrial Crops and Products, 124, 59-65.
[16]. Hatano, T., Edamatsu, R., Hiramatsu, M., Mori, A., Fujita, Y., Yasuhara, T., Yoshida, T., & Okuda, T., 1989, Effects of the interaction of tannins with co-existing substances. VI.: effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-Diphenyl-2-picrylhydrazyl radical. Chemical and Pharmaceutical Bulletin, 37(8), 2016-2021.
[17]. Padmanabhan, P., & Jangle, S. N., 2012, Evaluation of in-vitro anti-inflammatory activity of herbal preparation, a combination of four medicinal plants. International Journal of Basic and Applied Medical Sciences, 2(1), 109-116.
[18]. Elias, G., & Rao, M. N., 1988, Inhibition of albumin denaturation and antiinflammatory activity of dehydrozingerone and its analogs. Indian Journal of Experimental Biology, 26(7), 540–542.
[19]. Vajrabhaya, L. O., & Korsuwannawong, S., 2018, Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. Journal of Analytical Science and Technology, 9(1), 1-6.
[20]. Schmittgen, T. D., & Livak, K. J., 2008, Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols, 3(6), 1101–1108. https://doi.org/10.1038/nprot.2008.73.
[21]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P., & Jasmine, S., 2023, Unlocking the potential of beta-sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), 1007-1013.
[22]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P., & Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), 704-713.
[23]. Jayaraman, S., Natararaj, S., &Veeraraghavan, V. P., 2024, Hesperidin Inhibits Oral Cancer Cell Growth via Apoptosis and Inflammatory Signaling-Mediated Mechanisms: Evidence From In Vitro and In Silico Analyses. Cureus, 16(2).
[24]. Jayaraman, S., Veeraraghavan, V. P., Natarajan, S. R., & Jasmine, S., 2024, Exploring the therapeutic potential of curcumin in oral squamous cell carcinoma (HSC-3 cells): Molecular insights into hypoxia-mediated angiogenesis. Pathology-Research and Practice, 254, 155130.
Viewed PDF 207 11 -
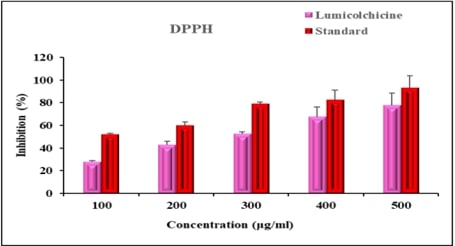 Molecular Approach to Identify Antitumorigenic Potential of Lumicolchicine in MCF-7 cells: Evidence Through Angiogenic SignallingAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.25.01.Art004
Molecular Approach to Identify Antitumorigenic Potential of Lumicolchicine in MCF-7 cells: Evidence Through Angiogenic SignallingAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.25.01.Art004Molecular Approach to Identify Antitumorigenic Potential of Lumicolchicine in MCF-7 cells: Evidence Through Angiogenic Signalling
Abstract:
Breast cancer remains a leading cause of mortality among women worldwide, highlighting the urgent need for new therapeutic agents. This study evaluates the cytotoxicity of Lumicolchicine (LMC) against the MCF-7 breast cancer cell line using both in vitro and in silico methods, with a focus on angiogenic signaling pathways. The in vitro assessment involved treating MCF-7 cells with varying concentrations of LMC and measuring cell viability using the MTT assay. Results indicated a dose-dependent reduction in cell proliferation, demonstrating LMC's cytotoxicity. To explore the molecular mechanisms underlying LMC's effects, we conducted in silico molecular docking studies on angiogenic signaling proteins: HIF1A, AKT, mTOR, VEGF, and ERK. The simulations revealed strong binding affinities of LMC to these targets, suggesting inhibition of angiogenic pathways crucial for tumor growth and metastasis. Further validation through quantitative PCR and Western blot analyses confirmed these findings, showing decreased expression levels of VEGF, VEGFR2, and HIF-1α in treated MCF-7 cells, supporting the notion that LMC suppresses angiogenesis. In summary, our combined in vitro and in silico findings suggest that Lumicolchicine has significant potential as an antitumor agent against breast cancer by targeting and inhibiting angiogenic signaling pathways. This study provides a foundation for future preclinical and clinical investigations into Lumicolchicine's use in breast cancer therapy.
Molecular Approach to Identify Antitumorigenic Potential of Lumicolchicine in MCF-7 cells: Evidence Through Angiogenic Signalling
References:
[1]. Łukasiewicz, S., Czeczelewski, M., Forma, A., Baj, J., Sitarz, R., & Stanisławek, A., 2021, Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers, 13(17), 4287. https://doi.org/10.3390/cancers13174287
[2]. Miziak, P., Baran, M., Błaszczak, E., Przybyszewska-Podstawka, A., Kałafut, J., Smok-Kalwat, J., Dmoszyńska-Graniczka, M., Kiełbus, M., & Stepulak, A., 2023, Estrogen Receptor Signaling in Breast Cancer. Cancers, 15(19), 4689. https://doi.org/10.3390/cancers15194689
[3]. Giatagana, E. M., Berdiaki, A., Tsatsakis, A., Tzanakakis, G. N., & Nikitovic, D., 2021, Lumican in Carcinogenesis-Revisited. Biomolecules, 11(9), 1319. https://doi.org/10.3390/biom11091319
[4]. Lee, H., & Kang, K. T., 2021, Differential angiogenic responses of human endothelial colony-forming cells to different molecular subtypes of breast cancer cells. Journal of lipid and atherosclerosis, 10(1), 111–122. https://doi.org/10.12997/jla.2021.10.1.111.
[5]. Shibuya M., 2011, Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes & cancer, 2(12), 1097–1105. https://doi.org/10.1177/1947601911423031
[6]. Miricescu, D., Totan, A., Stanescu-Spinu, I. I., Badoiu, S. C., Stefani, C., & Greabu, M., 2020, PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From molecular landscape to clinical aspects. International Journal of Molecular Sciences, 22(1), 173. https://doi.org/10.3390/ijms22010173
[7]. Madu, C. O., Wang, S., Madu, C. O., & Lu, Y., 2020, Angiogenesis in Breast Cancer Progression, Diagnosis, and Treatment. Journal of Cancer, 11(15), 4474–4494. https://doi.org/10.7150/jca.44313
[8]. Bui, B. P., Nguyen, P. L., Lee, K., & Cho, J., 2022, Hypoxia-Inducible Factor-1: A Novel Therapeutic Target for the Management of Cancer, Drug Resistance, and Cancer-Related Pain. Cancers, 14(24), 6054. https://doi.org/10.3390/cancers14246054
[9]. Jayaraman, S., Veeraraghavan, V. P., Natarajan, S. R., & Jasmine, S., 2024, Exploring the therapeutic potential of curcumin in oral squamous cell carcinoma (HSC-3 cells): Molecular insights into hypoxia-mediated angiogenesis. Pathology-Research and Practice, 254, 155130.
[10]. Krock, B. L., Skuli, N., & Simon, M. C., 2011, Hypoxia-induced angiogenesis: good and evil. Genes & Cancer, 2(12), 1117–1133. https://doi.org/10.1177/1947601911423654
[11]. Yang, F., Lee, G., & Fan, Y., 2024, Navigating tumor angiogenesis: therapeutic perspectives and myeloid cell regulation mechanism. Angiogenesis, 10.1007/s10456-024-09913-z. Advance online publication. https://doi.org/10.1007/s10456-024-09913-z
[12]. Saman, H., Raza, S. S., Uddin, S., & Rasul, K., 2020, Inducing Angiogenesis, a Key Step in Cancer Vascularization, and Treatment Approaches. Cancers, 12(5), 1172. https://doi.org/10.3390/cancers12051172
[13]. Liu, X., Hu, Y. J., Chen, B., Min, L., Peng, X. S., Zhao, J., Li, S., Wong, H. N. C., & Li, C. C., 2017, Asymmetric Total Syntheses of Colchicine, β-Lumicolchicine, and Allocolchicinoid N-Acetylcolchinol-O-methyl Ether (NCME). Organic Letters, 19(17), 4612–4615. https://doi.org/10.1021/acs.orglett.7b02224
[14]. Chen, S., Shen, X., Cheng, S., Li, P., Du, J., Chang, Y., & Meng, H., 2013, Evaluation of garlic cultivars for polyphenolic content and antioxidant properties. PloS One, 8(11), e79730. https://doi.org/10.1371/journal.pone.0079730
[15]. Khan, M. W. A., Otaibi, A. A., Alsukaibi, A. K. D., Alshammari, E. M., Al-Zahrani, S. A., Sherwani, S., Khan, W. A., Saha, R., Verma, S. R., & Ahmed, N., 2022, Biophysical, biochemical, and molecular docking investigations of anti-glycating, antioxidant, and protein structural stability potential of garlic. Molecules (Basel, Switzerland), 27(6), 1868. https://doi.org/10.3390/molecules27061868
[16]. Wu, X. X., Yue, G. G., Dong, J. R., Lam, C. W., Wong, C. K., Qiu, M. H., & Lau, C. B., (2018). actein inhibits the proliferation and adhesion of human breast cancer cells and suppresses migration in vivo. Frontiers in Pharmacology, 9, 1466. https://doi.org/10.3389/fphar.2018.01466
[17]. Jayaraman, S., Natararaj, S., & Veeraraghavan, V. P., 2024, hesperidin inhibits oral cancer cell growth via apoptosis and inflammatory signaling-mediated mechanisms: evidence from in vitro and in silico analyses. Cureus, 16(2), e53458. https://doi.org/10.7759/cureus.53458
[18]. Hagras, M., El Deeb, M. A., Elzahabi, H. S. A., Elkaeed, E. B., Mehany, A. B. M., & Eissa, I. H., 2021, Discovery of new quinolines as potent colchicine binding site inhibitors: design, synthesis, docking studies, and anti-proliferative evaluation. Journal of Enzyme Inhibition and Medicinal Chemistry, 36(1), 640–658. https://doi.org/10.1080/14756366.2021.1883598.
[19]. Pradeep, V. R., Menaka, S., Suresh, V., & Jayaraman, S., 2024, Anticancer Effects of Rosmarinus officinalis Leaf Extract on KB Cell Lines. Cureus, 16(2), e54031. https://doi.org/10.7759/cureus.54031.
[20]. Perumal, S., Langeshwaran, K., Selvaraj, J., Ponnulakshmi, R., Shyamaladevi, B., & Balasubramanian, M. P., 2018, Effect of diosmin on apoptotic signaling molecules in N-nitrosodiethylamine-induced hepatocellular carcinoma in experimental rats. Molecular And Cellular Biochemistry, 449(1-2), 27–37. https://doi.org/10.1007/s11010-018-3339-3.
Viewed PDF 196 14 -
 Effects of Hesperidin on Histopathological and Epigenetic Changes in Streptozotocin-Induced Type-2 Diabetic RatsAuthor: Ponnulakshmi RajagopalDOI: 10.21522/TIJPH.2013.SE.25.01.Art005
Effects of Hesperidin on Histopathological and Epigenetic Changes in Streptozotocin-Induced Type-2 Diabetic RatsAuthor: Ponnulakshmi RajagopalDOI: 10.21522/TIJPH.2013.SE.25.01.Art005Effects of Hesperidin on Histopathological and Epigenetic Changes in Streptozotocin-Induced Type-2 Diabetic Rats
Abstract:
Chemicals have been shown to induce epigenetic changes that alter glucose metabolism genes, potentially leading to insulin resistance and increasing the risk of metabolic disorders like type 2 diabetes. This study was aimed to assess histopathological and epigenetic changes in insulin signalling molecules in STZ-induced type-2 diabetic rats and the possible therapeutic role of hesperidin. Hesperidin (100mg/kg b.wt) was administered to STZ-induced rats and assessed for its protective role and epigenetic mechanisms in the gastrocnemius muscle. Diabetic rats exhibited significant increase (p<0.05) in renal function markers such as urea (60, 140, 80, 70, and 79 mg/dL) and creatinine (0.9, 2, 1.2, 1.1, and 1.0 mg/dL), oxidative stress markers, while antioxidant enzymes such as superoxide dismutase (0.9, 0.5,0.8, 0.87 and U/mg protein) and catalase (1, 0.4,0.86, 0.92 and 1.13 U/mg protein) were markedly lower (p<0.05). Histopathological analysis revealed a decrease and disruption in muscle fibres. The mRNA expression of insulin signalling molecules PI3K (1, 0.6, 0.8, 1.1, and 1 fold) and Munc18 (1, 0.6, 0.8, 1, and 0.9) was significantly (p<0.01) reduced in diabetic groups. Epigenetic studies showed CpG island methylation in the promoter regions of GLUT4, Akt, and IR genes in diabetic rats. However, hesperidin treatment restored the detrimental changes caused by diabetogenic agent, streptozotocin. The present study concludes that hesperidin plays a central role in regulating epigenetic mechanisms of insulin signalling molecules and GLUT4 translocation in skeletal muscle and thereby protects the muscle cells.
Effects of Hesperidin on Histopathological and Epigenetic Changes in Streptozotocin-Induced Type-2 Diabetic Rats
References:
[1]. Herman, W. H., 2017, The Global Burden of Diabetes: An Overview. 10.1007/978-3-319-41559-8_1.
[2]. Pyrzynska, K., 2022, Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients, 14(12), 2387. doi: 10.3390/nu14122387
[3]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P. and Jasmine, S., 2023. Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), pp.1007-1013.
[4]. Jayaraman, R., Subramani, S., Sheik Abdullah S. H., & Udaiyar M., 2018, Antihyperglycemic effect of hesperidin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomedicine and Pharmacotherapy, 97, 98-106. doi: 10.1016/j.biopha.2017.10.102.
[5]. Al-Ishaq, R. K., Abotaleb M., Kubatka P., Kajo K., & Büsselberg D., 2019, Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules, 9(9), 430. doi:10.3390/biom9090430.
[6]. Sundaram, R., Nandhakumar E., & Haseena B. H., 2019, Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats, Toxicology Mechanisms and Methods, 29:9, 644-653.
[7]. de Souza P, da Silva R. C. V., Mariano, L. N. B., Dick, S. L., & Ventura GC, Cechinel-Filho V, 2022, Diuretic and Natriuretic Effects of Hesperidin, a Flavanone Glycoside, in Female and Male Hypertensive Rats. Plants (Basel), 21, 12(1):25.
[8]. Sruthi, M. A., Mani, G., Ramakrishnan, M. and Selvaraj, J., 2023, Dental caries as a source of Helicobacter pylori infection in children: An RT‐PCR study. International Journal of Paediatric Dentistry, 33(1), pp.82-88.
[9]. Peng, P., Jin J., Zou G., Sui Y., Han Y, Zhao D., & Liu L., 2021. Hesperidin prevents hyperglycemia in diabetic rats by activating the insulin receptor pathway. Experimental and Therapeutic Medicine, 21(1):53.
[11]. Krishnan, R. P., Pandiar, D., Ramani, P. and Jayaraman, S., 2025, Molecular profiling of oral epithelial dysplasia and oral squamous cell carcinoma using next generation sequencing. Journal of Stomatology, Oral and Maxillofacial Surgery, 126(4), p.102120.
[12]. Mahmoud, A. M., 2016, Hesperidin as a Promising Anti-Diabetic Flavonoid: the Underlying Molecular Mechanism. Int Journal of Food and Nutritional Science, 3, 313-314.
[13]. Holliday, R., 2006, Epigenetics: a historical overview. Epigenetics, 1: 76– 80.
[14]. Weinhold. B., 2006, Epigenetics: the science of change. Environ Health Perspect, 114(3), A160-7.
[15]. Berger, S. L., Kouzarides, T., Shiekhattar, R., Shilatifard, A., 2009, An operational definition of epigenetics. Genes & Development, 23(7), 781-3. doi: 10.1101/gad.1787609.
[16]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P. and Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), pp.704-713.
[17]. Moosavi, A., Motevalizadeh A. A., 2016, Role of Epigenetics in Biology and Human Diseases. Iranian Biomedical Journal, 20(5), 246-58. doi: 10.22045/ibj.2016.01.
[18]. Kawada, J., 1992, New hypotheses for the mechanisms of streptozotocin and alloxan inducing Diabetes mellitus. Yakugaku Zasshi, 112(11), 773-91. Japanese. doi: 10.1248/ yakushi1947. 112.11_773
[19]. Szkudelski, T., 2001, The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological Research, 50(6):537-46.
[20]. Chen, S., Gan, D., Lin, S., Zhong, Y., Chen M., Zou X., Shao Z., & Xiao, G., 2022, Metformin in aging and aging-related diseases: clinical applications and relevant mechanisms. Theranostics, 12(6), 2722-2740. doi: 10.7150/thno.71360.
[21]. Gurina, T. S., Simms, L., Histology, Staining. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books /NBK557663/
[22]. Tahir, R. A., Zheng, D. A., Nazir, A., & Qing, H., 2019, A review of computational algorithms for CpG islands detection. Journal of Biosciences, 44(6), 143.
[23]. Patrick, P. L., Lam., Mitsuyo O., Subhankar D., Yu H., Tairan Q., Tao L., Dan Z., Youhou, K., Yunfeng, L., Maria, K., Li X., Wilson C. Y., et al., 2013, Munc18b Is a Major Mediator of Insulin Exocytosis in Rat Pancreatic β-Cells. Diabetes, 62(7), 2416–2428. https://doi.org/10.2337/db12-1380
[24]. Świderska E., Strycharz, J., Wróblewski, A., Szemraj J., Drzewoski J., & Śliwińska A., 2020, Role of PI3K/AKT Pathway in Insulin-Mediated Glucose Uptake [Internet]. Blood Glucose Levels. IntechOpen; 2020. http://dx.doi.org/10.5772/intechopen.80402
[25].Fathima, J. S., Jayaraman, S., Sekar, R. and Syed, N. H., 2024, The role of MicroRNAs in the diagnosis and treatment of oral premalignant disorders. Odontology, pp.1-10.
[26]. Ivorra, M. D., Paya, M., Villar, A., 1989, A review of natural products and plants as potential antidiabetic drugs. Journal of Ethnopharmacology, 27(3), 243–275.
[27]. Anil, B., 2010, Gaikwad, Jeena Gupta, Kulbhushan Tikoo; Epigenetic changes and alteration of Fbn1 and Col3A1 gene expression under hyperglycaemic and hyperinsulinaemic conditions. Biochemistry Journal, 432 (2), 333–341. doi: https://doi.org/10.1042/BJ20100414
[28]. Yasothkumar, D., Ramani, P., Jayaraman, S., Ramalingam, K. and Tilakaratne, W. M., 2024, Expression Profile of Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck Pathology, 18(1), p.28.
[29]. Bansal, A., & Pinney, S. E., 2017, DNA methylation and its role in the pathogenesis of diabetes. Pediatr Diabetes, 18(3),167-177. doi: 10.1111/pedi.12521.
[30]. Sagar, S., Ramani, P., Moses, S., Gheena, S. and Selvaraj, J., 2024, Correlation of salivary cytokine IL-17A and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology, pp.1-10.
[31]. Thomas, D. D., Corkey, B. E., Istfan N.W., & Apovian C. M., 2019, Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. Journal of the Endocrine Society, 3(9),1727-1747. doi: 10.1210/js.2019-00065.
[32]. Wilcox. G., 2005, Insulin and insulin resistance. Clinical Biochemist Reviews, 2005 26(2), 19-39.
[33]. Zhao, C., Yang C., Wai, S. T. C., Zhang Y. P., Portillo M, Paoli P., Wu Y., San Cheang W., Liu B., Carpéné C., Xiao J., & Cao H., 2019, Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus. Critical Reviews in Food Science and Nutrition; 59(6):830-847. doi: 10.1080/10408398.2018.1501658.
[34]. Stanley M. P. P., & Kamalakkannan N., 2006, Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. Journal of Biochemical and Molecular Toxicology, 20(2), 96-102. doi: 10.1002/jbt.20117.
[35]. Latha M, & Pari L. 2003, Antihyperglycaemic effect of Cassia auriculata in experimental diabetes and its effects on key metabolic enzymes involved in carbohydrate metabolism. Clinical and Experimental Pharmacology and Physiology, 30(1‐2):38-43.
[36]. Jayachandran, M., Zhang, T., Ganesan, K., Xu, B., Chung, & S.S.M. .2018, Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signaling pathway in streptozotocin-induced diabetic rats. European Journal of Pharmacology, 82:112–120.
[37]. Pari, L, & Rajarajeswari, N., 2009. Efficacy of coumarin on hepatic key enzymes of glucose metabolism in chemical induced type 2 diabetic rats. Chemico-Biological Interactions, 181:292–296.
[38]. Gothandam, K., Ganesan, V. S., Ayyasamy, T., & Ramalingam, S., 2019, Antioxidant potential of theaflavin ameliorates the activities of key enzymes of glucose metabolism in high fat diet and streptozotocin–induced diabetic rats. Redox Report, 24:41–50.
[39]. Tian, M., Han, Y. B., Zhao, C. C., et al. 2021, Hesperidin alleviates insulin resistance by improving HG-induced oxidative stress and mitochondrial dysfunction by restoring miR-149. Diabetol Metab Syndr 13:50. https://doi.org/10.1186/s13098-021-00664-1.
[40]. Chen C, Peng S, Chen F, Liu L, Li Z, Zeng G, Huang Q. 2017, Protective effects of pioglitazone on vascular endothelial cell dysfunction induced by high glucose via inhibition of IKKα/β-NFκB signaling mediated by PPARγ in vitro. Canadian Journal of Physiology and Pharmacology, 95(12), 1480-1487. doi: 10.1139/cjpp-2016-0574.
[41]. Copps, K.D.;,White, M.F. 2012, Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia, 55:2565–2582.
[42]. Chuang, W.T., Yen, C.C., Huang, C.S., Chen, H.W., Lii, C. K., 2020, Benzyl Isothiocyanate Ameliorates High-Fat Diet-Induced Hyperglycemia by Enhancing Nrf2-Dependent Antioxidant Defense-Mediated IRS-1/AKT/TBC1D1 Signaling and GLUT4 Expression in Skeletal Muscle. Journal of Agricultural and Food Chemistry, 2020, 68, 15228–15238.
[43]. Boucher, J., Kleinridders, A., Kahn, C. R., 2014, Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harbor Perspectives in Biology, 2014, 6, a009191.
[44]. Zhang, Y. Xu, W., Huang, X., Zhao, Y., Ren, Q., Hong, Z., Huang, M., Xing, X., 2018, Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic rats partially through IRS-1/PI3K/Akt and AMPK pathways. Journal of Functional Foods, 48, 515–524.
[45]. Cai, S., Sun, W., Fan, Y., Guo, X., Xu, G., Xu, T., Hou, Y., Zhao, B., Feng, & X., Liu, 2016. T. Effect of mulberry leaf (Folium Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling pathway in type 2 diabetes mellitus rats. Pharmaceutical Biology, 54, 2685–2691.
[46]. Hsu, W. H., Hsiao, P. J., Lin, P. C., Chen, S. C., Lee, M. Y., Shin, S. J., 2017. Effect of metformin on kidney function in patients with type 2 diabetes mellitus and moderate chronic kidney disease. Oncotarget, 9(4), 5416-5423. doi: 10.18632/oncotarget.23387.
[47]. Bandeira Sde, M, Guedes Gda, S, da Fonseca, L. J., Pires, A. S., Gelain, D. P., Moreira J. C., Rabelo, L. A., Vasconcelos, S. M., Goulart, M. O., 2012, Characterization of blood oxidative stress in type 2 diabetes mellitus patients: increase in lipid peroxidation and SOD activity. Oxid Med Cell Longev, 819310. doi: 10.1155/2012/819310.
Viewed PDF 234 14 -
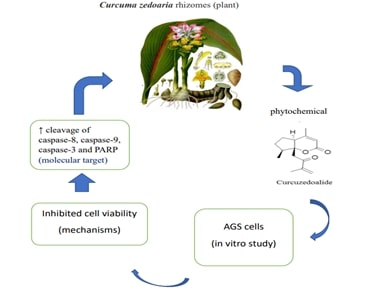 Therapeutic Implications of Medicinal Plants in Combatting Gastric CancerAuthor: Ponnulakshmi RDOI: 10.21522/TIJPH.2013.SE.25.01.Art006
Therapeutic Implications of Medicinal Plants in Combatting Gastric CancerAuthor: Ponnulakshmi RDOI: 10.21522/TIJPH.2013.SE.25.01.Art006Therapeutic Implications of Medicinal Plants in Combatting Gastric Cancer
Abstract:
Cancer ranks as the second most prevalent cause of mortality globally. More specifically, gastric cancer holds the second position in terms of cancer-related fatalities and is the fourth most frequently diagnosed cancer worldwide. A malignant condition called gastric carcinoma begins in the stomach. Despite decreased incidence, all malignancies continue to be second leading cause mortality around the globe. The majority of the stomach cancer aren’t discovered until they’re become rather large or have migrated outside the stomach in nations where routine screening for the disease is not practised. Loss of appetite, weight loss, stomach pain, feeling of fullness after eating a small amount. The Epstein- barr virus, in addition to H.pylori infection, is the second component linked to the development of GC. The treatment of gastric cancer is a complex process that typically involves in surgery, chemotherapy, radiation therapy, and targeted therapies. While plants and natural compounds have been explored for their potential in cancer treatment, it's important to note that there is no single plant or herbal remedy that can serve as a standalone cure for gastric cancer. Instead, various plants and their derivatives may play supportive roles in managing symptoms, improving the overall well-being of patients, and potentially enhancing the effectiveness of conventional treatments. In this review mainly focus of the medicinal plant such as Curcuma Mangga Rhizomes, Curcuma Zedoaria Rhizomes, Zanthoxylum Nitidum, Perilla Frutescens, Bamboo Shavings, Hericium Erinaceus Mycelium, Liang Jing mushroom, Turmeric.
Therapeutic Implications of Medicinal Plants in Combatting Gastric Cancer
References:
[1]. Sitarz, R., Skierucha, M., Mielko, J., Offerhaus, G. J. A., Maciejewski, R., Polkowski, W. P., 2023, Gastric cancer: epidemiology, prevention, classification, and treatment, 239-248 10.2147/CMAR.S149619.
[2]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P., & Jasmine, S. 2023, Unlocking the potential of beta-sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), 1007-1013.
[3]. Alipour, M., 2021, Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J Gastrointest Cancer, 23-30.10.1007/s12029-020-00518-5.
[4]. Sruthi, M. A., Mani, G., Ramakrishnan, M. and Selvaraj, J., 2023, Dental caries as a source of Helicobacter pylori infection in children: An RT‐PCR study. International Journal of Paediatric Dentistry, 33(1), pp.82-88.
[5]. Mao, Q. Q., Xu, X. Y., Shang, A., Gan, R. Y., Wu, D. T., Atanasov, A. G., Li, H. B., 2020, Phytochemicals for the Prevention and Treatment of Gastric Cancer: Effects and Mechanisms, 10.3390/ijms21020570.
[6]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P. and Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), pp.704-713.
[7]. Mustapha, N. M., 2010, ASEAN herbal and medicinal plants ASEAN Secretariat, Jakarta, Indonesia, 336(p).
[8]. Krishnan, R. P., Pandiar, D., Ramani, P. and Jayaraman, S., 2025, Molecular profiling of oral epithelial dysplasia and oral squamous cell carcinoma using next generation sequencing. Journal of Stomatology, Oral and Maxillofacial Surgery, 126(4), p.102120.
[9]. Jung, E. B., Trinh, T. A., Lee, T. K., Yamabe, N., Kang, K. S., Song, J. H., Choi, S., Lee, S., Jang, T. S., Kim, K. H., Hwang, G. S., 2018, Curcuma zedoalide contributes to the cytotoxicity of Curcuma zedoaria rhizomes against human gastric cancer AGS cells through induction of apoptosis, 213, 48–55. 10.1016/j.jep.2017.10.025.
[10]. Eun Bee Jung, Tuy An Trinh, Kyoung Lee, et al.: Curcuma zedoalide contributes to the cytotoxicity of Curcuma zedoaria rhizomes against human gastric cancer AGS cells through induction of apoptosis Journal of Ethnopharmacology. 2018, 48-55. 10.1016/j.jep.2017.10.025
[11]. Salvesen, G. S., Dixit, V. M., 1997, Caspases: intracellular signalling by proteolysis, 443-446. 10.1016/s0092-8674(00)80430-4.
[12]. Li, P., Nijhawan, D., Budihardjo, I., Srinivasula, S. M., Ahmad, M., Alnemri, E. S., Wang, X., 1997, Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade, 479-489. 10.1016/s0092-8674(00)80434-1.
[13]. Earnshaw, W. C., Martins, L. M., Kaufmann, S. H., 1999, Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annual Review of Biochemistry, 383-424. 10.1146/annurev.biochem.68.1.383.
[14]. Broker, L. E., Kruyt, F. A., Giaccone, G., 2005, Cell death independent of caspases: a review, 10.1158/1078-0432.ccr-04-2223.
[15]. Zhang, C. C., Cao, C. Y., Kubo, M., Harada, K., Yan, X. T., Fukuyama, Y., Gao, J. M., 2017, Chemical Constituents from Hericium erinaceus Promote Neuronal Survival and Potentiate Neurite Outgrowth via the TrkA/Erk1/2 Pathway, 10.3390/ijms18081659.
[16]. Kuo, H. C., Kuo, Y. R., Lee, K. F., Hsieh, M. C., Huang, C. Y., Hsieh, Y. Y., Lee, K. C., Kuo, H. L., Lee, L. Y., Chen, W. P., Chen, C. C., Tung, S. Y., 2017, A Comparative Proteomic Analysis of Erinacine a’s Inhibition of Gastric Cancer Cell Viability and Invasiveness, 195-208, 10.1159/000480338
[17]. Cordeiro, Y., Machado, F., Juliano, L., Juliano, M. A., Brentani, R. R., Foguel, D., 2001, Journal of Biological Chemistry, 276(24), 21887–21893.
[18]. Sagar, S., Ramani, P., Moses, S., Gheena, S. and Selvaraj, J., 2024, Correlation of salivary cytokine IL-17A and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology, pp.1-10.
[19]. Schaller, M. D., 2004, FAK and paxillin regulators of N-cadherin adhesion and inhibitors of cell migration, 157-169. 10.1083/jcb.200406151.
[20]. Manning, B. D., Cantley, L. C., 2007, AKT/PKB signalling: navigating downstream, 1261-1274. 10.1016/j.cell.2007.06.009.
[21]. Weng, Q. P., Kozlowski, M., Belham, C., Zhang, A., Comb, M. J., Avruch, J., 1998, Regulation of the p70 S6 kinase by phosphorylation In vivo. Analysis using site-specific anti-phosphopeptide antibodies, 16621-16629. 10.1074/jbc.273.26.16621
[22]. Bokoch, G. M., 2003, Biology of the p21-activated kinases. Annu Rev Biochem, 743-781. 10.1146/annurev.biochem.72.121801.161742
[23]. Liu, Y. B., Nair, M. G., 2011, Labdane diterpenes in Curcuma mangga rhizomes inhibit lipid peroxidation, cyclooxygenase enzymes and human tumour cell proliferation. Food Chem, 124, 527–532. 10.1016/j.foodchem.2010.06.064
[24]. Malek, S. N., Lee, G. S., Hong, S. L., Yaacob, H., Wahab, N. A., Faizal Weber, J. F., Shah, S. A., 2011, Phytochemical and cytotoxic investigations of Curcuma mangga rhizomes Molecules, 4539-4548. 10.3390/molecules16064539
[25]. Liu, Y., Nair, M., 2012, Curcuma longa and Curcuma mangga leaves exhibit functional food property Food Chem, 135 (2), 634-640. 10.1016/j.foodchem.2012.04.129
[26]. Keum, Y. W., 2011, Differential Modulation of Helicobacter pylori Drug Susceptibility by Specific Fatty Acids," Antimicrobial Agents and Chemotherapy, 2867-2875. 10.1128/AAC.01432-10
[27]. Yunbao Liu, Muraleedharan G. Nair: Labdane diterpenes in Curcuma mangga rhizomes inhibit lipid peroxidation, cyclooxygenase enzymes and human tumour cell proliferation. 2011.10.1016/j.foodchem.2010.06.064
[28]. Xu, Q., Li, Z. X., Ye, Z. M., 2011, Nitidine chloride-induced apoptosis of human osteosarcoma cells and its mechanism]. Nan Fang Yi Ke Da Xue Xue Bao. PMID: 21354931
[29]. Hu, J., Zhang, W. D., Liu, R. H., Zhang, C., Shen, Y. H., Li, H. L., Liang, M. J., Xu, X. K., 2006, Benzophenanthridine alkaloids from Zanthoxylum nitidum (Roxb) DC, and their analgesic and anti-inflammatory activities, 10.1002/cbdv.200690108.
[30]. An, R., Hou, Z., Li, J. T., Yu, H. N., Mou, Y. H., Guo, C., 2018, Synthesis and Biological Evaluation of Novel 4-Substituted Coumarin Derivatives as Antitumor Agent,10.3390/molecules23092281.
[31]. Yasothkumar, D., Ramani, P., Jayaraman, S., Ramalingam, K. and Tilakaratne, W.M., 2024, Expression Profile of Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck Pathology, 18(1), p.28.
[32]. Yang, Y., Cao, Y., Chen, L., Liu, F., Qi, Z., Cheng, X., Wang, Z., 2018, Cryptotanshinone suppresses cell proliferation and glucose metabolism via STAT3/SIRT3 signaling pathway in ovarian cancer," Journal of Cellular Physiology, 10.1002/cam4.1691.
[33]. Chen, J., Wang, J., Lin, L., He, L., 2011, Inhibition of STAT3 Signaling Pathway by Nitidine Chloride Suppressed the Angiogenesis and Growth of Human Gastric Cancer, 10.1158/1535-7163.MCT-11-0648
[34]. Cui, Y., Lai, B., Tang, X., 2019, Microbial Fuel Cell-Based Biosensors, 10.3390%2Fbios9030092
[35]. Gao, Y., Lyu, L., Feng, Y., Li, F., Hu, Y., 2021, A review of cutting-edge therapies for hepatocellular carcinoma (HCC): Perspectives from patents, 3066-3081. 10.7150/ijms.59930.
[36]. Zhang, Y., Liu, S. S., Feng, Q., Huang, X., Wang, X., Peng, Y., Zhao, Z., Liu, Z., 2019, Perillaldehyde activates AMP-activated protein kinase to suppress the growth of gastric cancer via induction of autophagy, 1716–1725. 10.1002/jcb.27491
[37]. Pazhani, J., Chanthu, K., Jayaraman, S. and Varun, B. R., 2023, Evaluation of salivary MMP-9 in oral squamous cell carcinoma and oral leukoplakia using ELISA. Journal of Oral and Maxillofacial Pathology, 27(4), pp.649-654.
[38]. Lu, B. Y., Wu, X. Q., Tie, X. W., Zhang, Y., Zhang, Y., 2005, Toxicology and safety of anti-oxidant of bamboo leaves. Part 1: Acute and subchronic toxicity studies on anti-oxidant of bamboo leaves. Food Chem Toxicol, 783–792. 10.1016/j.fct.2005.01.019.
[39]. Fathima, J. S., Jayaraman, S., Sekar, R. and Syed, N. H., 2024, The role of MicroRNAs in the diagnosis and treatment of oral premalignant disorders. Odontology, pp.1-10.
[40]. Gong, J. Y., Wu, X. Q., Lu, B. Y., Zhang, Y., 2010, Safety evaluation of polyphenol-rich extract from bamboo shavings. Afr J Biotechnol, 77–86. http://www.academicjournals.org/AJB
[41]. Sreevarun, M., Ajay, R., Suganya, G., Rakshagan, V., Bhanuchander, V., & Suma, K., 2023, Formulation, Configuration, and Physical Properties of Dental Composite Resin Containing a Novel 2π + 2π Photodimerized Crosslinker - Cinnamyl Methacrylate: An In Vitro Research. The Journal Of Contemporary Dental Practice, 24(6), 364–371. https://doi.org/10.5005/jp-journals-10024-3480
[42]. Alam, M. K., Alqhtani, N. R., Alnufaiy, B., Alqahtani, A. S., Elsahn, N. A., Russo, D., Di Blasio, M., Cicciù, M., & Minervini, G., 2024, A systematic review and meta-analysis of the impact of resveratrol on oral cancer: potential therapeutic implications. BMC Oral Health, 24(1), 412. https://doi.org/10.1186/s12903-024-04045-8
[43]. Yadalam, P. K., Arumuganainar, D., Ronsivalle, V., Di Blasio, M., Badnjevic, A., Marrapodi, M. M., Cervino, G., & Minervini, G., 2024, Prediction of interactomic hub genes in PBMC cells in type 2 diabetes mellitus, dyslipidemia, and periodontitis. BMC Oral Health, 24(1), 385. https://doi.org/10.1186/s12903-024-04041-y
[44]. Sun, Y. Q., Guo, T. K., Xi, Y. M., Chen, C., Wang, J., Wang, Z. R., 2007, Effects of AZT and RNA-protein complex (FA-2-b-beta) extracted from Liang Jin mushroom on apoptosis of gastric cancer cells, 10.3748/wjg.v13.i31.4185
[45]. Olivas-Aguirre F. J, Rodrigo-Garcia J, Martinez-Ruiz N. D. R., Cardenas- Robles, A. I., Mendoza-Diaz, S. O., Alvarez-Parrilla, E., Gonzalez-Aguilar, G. A., De la rosa, L. A., Ramos- Jimenez, A., Wall-Medrano, A., 2016, Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules, 10.3390/molecules21091264
[46]. Ashrafizadeh, M., Zarrabi, A., Hashemi, F., Zabolian, A., Saleki, H., Bagherian, M., Azami, N., Bejandi, K. A., Hushmandi, K., Ang, H. A., Makvandi, P., Khan, H., Kumar, A. P., 2020, Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms Enhancing Antitumor Activity, 10.3390/pharmaceutics12111084
[47]. Yu, L. L., Wu, J. G., Dai, N., Yu, H. G., Si, J. M., 2011, Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-κB transcription factor. 26, 1197–1203. 10.3892/or.2011.1410
Viewed PDF 260 10 -
 Evaluating Antioxidant and Antimicrobial Potential of Albizia saman Extract Against Candida albicansAuthor: Ponnulakshmi RDOI: 10.21522/TIJPH.2013.SE.25.01.Art007
Evaluating Antioxidant and Antimicrobial Potential of Albizia saman Extract Against Candida albicansAuthor: Ponnulakshmi RDOI: 10.21522/TIJPH.2013.SE.25.01.Art007Evaluating Antioxidant and Antimicrobial Potential of Albizia saman Extract Against Candida albicans
Abstract:
Albizia saman is a tree of the Fabaceae family that has been used for ethnomedical purposes since olden times. Prior investigations have reported probable medicinal value against a wide array of ailments, which may be attributed to its varied phytochemical composition. Thence, there is a requirement for comprehensive studies on its efficacy against individual pathogens and their mechanisms. The present study is an effort to encompass a comprehensive description of the antimicrobial, anti-inflammatory, and antioxidant potential of A. saman extract with major emphasis against Candida albicans. Various microbial methods have been used for the determination of the antimicrobial potential of A. saman extract, including disc diffusion, well diffusion, streak plate, and various dilution techniques. The anti-inflammatory and antioxidant activities were assayed in vitro and in vivo by various models. A. saman extract exhibited significant antimicrobial activity against the tested pathogens, C. albicans. It also potent anti-inflammatory and anti-oxidant activity. Phytochemical screening for A. The phytochemical screening of the leaf extract of Saman revealed several important phytochemicals: tannins, alkaloids, carbohydrates, saponins, flavonoids, protein, phenol, and ninhydrin. In view of the antimicrobial, anti-inflammatory and antioxidant properties of the extracts of A. saman, it holds immense potential in the development of new therapeutic agents. Findings of the present study clearly show that Albizia saman could be utilized to reveal the traditional uses of this plant, and to discover new therapeutic uses.
Evaluating Antioxidant and Antimicrobial Potential of Albizia saman Extract Against Candida albicans
References:
[1]. Vinodhini, S. & Devi Rajeswari, V., 2018, Review on Ethnomedical Uses, Pharmacological Activity and Phytochemical Constituents of Samanea saman(jacq.) Merr. Rain Tree. 10(2):202-209.
[2]. Sharifi-Rad, J., Hoseini-Alfatemi, S. M., Sharifi-Rad, M. & Teixeira da Silva, J. A., 2015, Antibacterial, antioxidant, antifungal and anti-inflammatory activities of crude extract from Nitraria schoberi fruits, https://doi.org/10.1007%2Fs13205-014-0266-1
[3]. Gonelimali, F. D., Lin, J., Miao, W., Xuan, J., Charles, F., Chen, M., Hatab, S. R., 2018, Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms, https://doi.org/10.3389/fmicb.2018.01639
[4]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P. and Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), pp.1007-1013.
[5]. Jovic, M. D., Agatonovic-Kustrin, S., Ristivojevic, P. M., Trifkovic, J. D. & Morton, D. W., 2023, Bioassay-Guided Assessment of Antioxidative, Anti-Inflammatory and Antimicrobial Activities of Extracts from Medicinal Plants via High-Performance Thin-Layer Chromatography, https://doi.org/10.3390/molecules28217346
[6]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P. and Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), pp.704-713.
[7]. Sruthi, M. A., Mani, G., Ramakrishnan, M. and Selvaraj, J., 2023, Dental caries as a source of Helicobacter pylori infection in children: An RT‐PCR study. International Journal of Paediatric Dentistry, 33(1), pp.82-88.
[8]. Krishnan, R. P., Pandiar, D., Ramani, P. and Jayaraman, S., 2025, Molecular profiling of oral epithelial dysplasia and oral squamous cell carcinoma using next generation sequencing. Journal of Stomatology, Oral and Maxillofacial Surgery, 126(4), p.102120.
[9]. Harborne, J. B., 1973, Phytochemical methods: A guide to modern techniques of plant analysis, 279–19.
[10]. Bhalodia, N. R. & Shukla, V. J., 2011, Antibacterial and antifungal activities from leaf extracts of Cassia fistula, An ethnomedicinal plant, https://doi.org/10.4103/2231-4040.82956
[11]. Khandelwal, K. R., 2009, Practical Pharmacognosy. Nirali Prakashan, 2nd ed. pp. 149-56. 26.
[12]. Kokate C. K. Practical Pharmacognosy. Delhi: New Gyan Offset Printers, 2000. pp. 107-9.
[13]. Mutharaian, N., Sasikumar, J. M., Pavai, P., Bai, V. N., 2009, In vitro antioxidant activity of Pterocarpus marsupium Roxb Leaves. Int J Biomed Pharma Sci 3:29-33.
[14]. Fratianni, F., Acierno, A. D., Ombra, M. N., 2021, Fatty Acid Composition, Antioxidant, and in vitro Anti-inflammatory Activity of Five Cold-Pressed Prunus Seed Oils, and Their Anti-biofilm Effect Against Pathogenic Bacteria. https://doi.org/10.3389/fnut.2021.775751
[15]. Gizaw, A., Marami, L. M., Teshome, I., Sarba, E. J., Admasu, P., Babele, D. A., Dilba, G. M., Bune, W. M., Bayu, M. D., Tadesse, M., & Abdisa, K., 2022, Phytochemical Screening and In Vitro Antifungal Activity of Selected Medicinal Plants against Candida albicans and Aspergillus niger in West Shewa Zone, Ethiopia. Advances in Pharmacological and Pharmaceutical Sciences, 2022, 3299146. https://doi.org/10.1155/2022/3299146
[16]. Krishnan, R. P., Pandiar, D., Ramani, P. and Jayaraman, S., 2025, Molecular profiling of oral epithelial dysplasia and oral squamous cell carcinoma using next generation sequencing. Journal of Stomatology, Oral and Maxillofacial Surgery, 126(4), p.102120.
[17]. Thangathirupathi, A., 2014, Evaluation of Anti-Diabetic Activity of Samanea saman (Jacq.) Merr. International Journal of Research in Pharmaceutical and Nano Sciences, 3(4): pp. 352-356.
[18]. Alam, M. K., Hossain, M. S., Hossain, M. A., & Khalil, M. I., 2020, Chapter 8: Acacia nilotica, Albizia saman, Azadirachta indica: Ethnobotany and Medicinal Uses. In Medicinal Plants and Sustainable Development, pp. 159-178). Springer, Singapore.
[19]. Fathima, J. S., Jayaraman, S., Sekar, R., & Syed, N. H. 2024, The role of MicroRNAs in the diagnosis and treatment of oral premalignant disorders. Odontology, 1-10.
[20]. Vinodhini, S., Sankar Ganesh, P., Arumugam, M., & Ramesh Babu, N. G., 2014, An overview on the biological perspectives of Samanea saman (Jacq.) Merr. International Journal of Pharmacy and Pharmaceutical Sciences, 6(5): 47-51.
[21]. Ferdous, A., Chowdhury, J. A., Rashid, M. A., & Malik, S. M. A., 2012, Evaluation of antioxidant and antimicrobial activities of Samanea saman (Jacq.) Merr. Pharmacognosy Journal, 4(32): 53-58.
[22]. Yasothkumar, D., Ramani, P., Jayaraman, S., Ramalingam, K., & Tilakaratne, W. M. 2024, Expression Profile of Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck Pathology, 18(1), 28.
[23]. Azhar, I. M., Hasan, M. M., & Mazhar, F. A., 2009, Some biological evaluations of Samanea Saman. Pakistan Journal of Pharmacology, 26: 47-53.
[24]. Arumugam, S., Selvaraj, S. V., Velayutham, S., Natesan, S. K., & Palaniswamy, K., 2011, Evaluation of anti-ulcer activity of Samanea Saman (Jacq.) Merr bark on ethanol and stress-induced gastric lesions in albino rats. Indian Journal of Pharmacology, 43(5), 585-590.
[25]. Ramalingam, K., Yadalam, P. K., Ramani, P., Krishna, M., Hafedh, S., Badnjević, A., Cervino, G., & Minervini, G., 2024, Light gradient boosting-based prediction of quality of life among oral cancer-treated patients. BMC oral health, 24(1), 349. https://doi.org/10.1186/s12903-024-04050-x
[26]. Thangathirupathi, A., 2014, Evaluation of Anti-Diabetic Activity of Samanea Saman (Jacq.) Merr. International Journal of Research in Pharmaceutical and Nano Sciences, 3(4), 352-356.
[27]. Girish Gulab Meshram, Anil Kumar, Waseem Rizvi, Tripathi, C. D., & Khan, R. K., 2016, Evaluation of the anti-inflammatory activity of the aqueous and ethanolic extracts of the leaves of Albizzia lebbeck in rats. Journal of Traditional and Complementary Medicine, 6(2), 172-175.
[28]. Neralla, M., M, H., Preethi, A., Selvakumar, S. C., & Sekar, D. 2024, Expression levels of microRNA-7110 in oral squamous cell carcinoma. Minerva dental and oral science, 73(3), 155–160. https://doi.org/10.23736/S2724-6329.23.04801-5.
[29]. Sagar, S., Ramani, P., Moses, S., Gheena, S. and Selvaraj, J., 2024, Correlation of salivary cytokine IL-17A and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology, pp.1-10.
[30]. Harsha, L., & Subramanian, A. K., 2022, Comparative Assessment of pH and Degree of Surface Roughness of Enamel When Etched with Five Commercially Available Etchants: An In Vitro Study. The journal of contemporary dental practice, 23(2), 181–185.
Lovett, J., & Ryuntyu, M., 1992, Allelopathy: Broadening the context. In Allelopathy: Basic and applied aspects, 11-19
Viewed PDF 272 8 -
 Asarone Possesses Antiproliferative Potential in Breast Cancer Cell Line (MCF-7) Through Via Apoptosis and Inflammatory-Mediated Signaling PathwaysAuthor: Selvaraj JayaramanDOI: 10.21522/TIJPH.2013.SE.25.01.Art008
Asarone Possesses Antiproliferative Potential in Breast Cancer Cell Line (MCF-7) Through Via Apoptosis and Inflammatory-Mediated Signaling PathwaysAuthor: Selvaraj JayaramanDOI: 10.21522/TIJPH.2013.SE.25.01.Art008Asarone Possesses Antiproliferative Potential in Breast Cancer Cell Line (MCF-7) Through Via Apoptosis and Inflammatory-Mediated Signaling Pathways
Abstract:
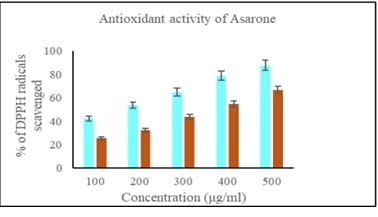
Breast cancer is a significant global health challenge, requiring continuous exploration of new treatments. Asarone, a bioactive compound from the Acorus genus, shows promising anticancer properties but its effects on breast cancer cells are underexplored. This study investigates asarone's anticancer potential against breast cancer cell lines using in vitro and in silico approaches. Asarone's antioxidant activity was evaluated using DPPH radical scavenging assays, revealing a dose-dependent (25.56, 32.18, 47.73, 54.83 and 66.74%) effect on free radicals. MTT assays showed a dose-dependent decrease in cell viability, indicating asarone's cytotoxicity towards breast cancer cells. mRNA expression analysis showed that targeting apoptosis regulators such as Bax (1, 1.3, 1.52 fold change upregualtion) and Bad (1, 1.4, and 1.6 fold upregulation) gene expression demonstrated that asarone induces apoptosis via the intrinsic pathway. Additionally, asarone inhibited Akt mNRA (1, 0.6, and 0.4 fold change down regulation), caspase-3 (1, 1.4, and 1.7 upregulation) and cytochrome-c mRNA (1, 1.2 and 1,54 fold change upregulation) suggesting interference with key cancer progression pathways. Molecular docking studies predicted favorable binding interactions between asarone and crucial proteins involved in apoptosis and cell survival, including Bax, Bad, cytochrome c, caspase 3, and Akt. These findings collectively highlight the multifaceted anticancer mechanisms of asarone against breast cancer cells. This study underscores the potential of asarone as a natural therapeutic agent for breast cancer, offering avenues for further exploration in translational research and clinical trials. The current study significantly advances our understanding of asarone's anticancer properties, offering promising directions for developing new and effective breast cancer therapies.
Asarone Possesses Antiproliferative Potential in Breast Cancer Cell Line (MCF-7) Through Via Apoptosis and Inflammatory-Mediated Signaling Pathways
References:
[1]. Alkabban, F. M., Ferguson, T., 2022, Breast Cancer. In: StatPearls. Treasure Island (FL): StatPearls Publishing; September 26.
[2]. Jayaraman, S., Raj Natarajan, S., Ponnusamy, B., Veeraraghavan, V.P., Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. Saudi Dent J,35(8):1007-1013. doi: 10.1016/j.sdentj.2023.08.003.
[3]. Janani, K. S., Gayatri Devi, R., Selvaraj, J., 2022, Antiproliferative effect of Merremia emarginata (Burm. F.) leaf extract on SAOS-2 cell line. J Pharm Negat Results, 13:1805-1810. doi:10.47750/pnr.2022.13.S06237.
[4]. JinJin, P., Yuqiang, Y., Selvaraj, J., Ponnulakshmi, R., Prabhu Manickam, N,, Vidhya Rekha, U, Sridevi, G., Jeane Rebecca, R., Janaki, C. S., Dwarakesh, T., Chella Perumal., Monica, M., 2024, A review on advancements in the application of starch-based nanomaterials in biomedicine: Precision drug delivery and cancer therapy. International Journal of Biological Macromolecules, 26(1):130746.,https://doi.org/10.1016/j.ijbiomac.2024.130746.
[5]. Benedict, A., Suresh, V., Muthamizh, S., Jayaraman, S., & Hussein, M. A., 2024, Merremia emarginata Extract Potentiating the Inhibition of Human Colon Cancer Cells (HT-29) via the Modulation of Caspase-3/Bcl-2 Mediated Pathways. Curēus, https://doi.org/10.7759/cureus.56300.
[6]. Roy, J. R., Janaki, C. S., Jayaraman, S., Veeraraghavan, V. P., Periyasamy, V., Balaji, T., Vijayamalathi, M., Bhuvaneswari, P., Swetha, P., 2023, Hypoglycemic Potential of Carica Papaya in Liver Is Mediated through IRS-2/PI3K/SREBP-1c/GLUT2 Signaling in High-Fat-Diet-Induced Type-2 Diabetic Male Rats. Toxics, 11(3):240. doi: 10.3390/toxics11030240.
[7]. Butti, R., Das, S., Gunasekaran, V.P., Yadav, A.S., Kumar, D., Kundu, G.C., 2018, Receptor tyrosine kinases (RTKs) in breast cancer: signaling, therapeutic implications and challenges. Mol Cancer,17(1):34. doi:10.1186/s12943-018-0797-x.
[8]. Chellian, R., Pandy, V., Mohamed, Z., 2017, Pharmacology and toxicology of α- and β-Asarone: A review of preclinical evidence. Phytomedicine, 32:41-58. doi:10.1016/j.phymed.2017.04.003
[9]. Hatano, T., Edamatsu, R., Hiramatsu, M., MORI, A., Fujita, Y., Yasuhara, T., OKUDA, T., 1989, Effects of the interaction of tannins with co-existing substances. VI.: effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-Diphenyl-2-picrylhydrazyl radical. Chemical and Pharmaceutical Bulletin, 37(8):2016-2021.
[10]. Perumal, S., Langeshwaran, K., Selvaraj, J., Ponnulakshmi, R., Shyamaladevi, B., Balasubramanian, M. P., 2018, Effect of diosmin on apoptotic signaling molecules in N-nitrosodiethylamine-induced hepatocellular carcinoma in experimental rats. Molecular and Cellular Biochemistry, 449: 27-37.
[11]. Zhang, X., Abdelrahman, A., Vollmar, B., & Zechner, D., 2018, The Ambivalent Function of YAP in Apoptosis and Cancer. International journal of molecular sciences, 19(12), 3770. https://doi.org/10.3390/ijms19123770.
[12]. Ramalingam, K., Yadalam, P. K., Ramani, P., Krishna, M., Hafedh, S., Badnjević, A., Cervino, G., & Minervini, G., 2024, Light gradient boosting-based prediction of quality of life among oral cancer-treated patients. BMC oral health, 24(1), 349. https://doi.org/10.1186/s12903-024-04050-x
[13]. Neralla, M., M, H., Preethi, A., Selvakumar, S. C., & Sekar, D., 2024, Expression levels of microRNA-7110 in oral squamous cell carcinoma. Minerva Dental and Oral Science, 73(3), 155–160. https://doi.org/10.23736/S2724-6329.23.04801-5
[14]. Prasad, M., Jayaraman, S., Rajagopal, P., Veeraraghavan, V. P., Kumar, P. K., Piramanayagam, S., Pari, L., 2022, Diosgenin inhibits ER stress-induced inflammation in aorta via iRhom2/TACE mediated signaling in experimental diabetic rats: An in vivo and in silico approach. Chem Biol Interact,358:109885. doi: 10.1016/j.cbi.2022.109885.
[15]. Kumar Subramanian, Aravind & Katyal, Deepika., 2023, The Effect of Topical Melatonin Gel on the oral health and salivary nickel and chromium content of orthodontic Patients: An In Vivo Study. World Journal of Dentistry. 14. 326-330. 10.5005/jp-journals-10015-2218.
[16]. Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., Veeraraghavan, V. P., 2022, Effect of Carica papaya on IRS-1/Akt signaling mechanisms in High-Fat-Diet–Streptozotocin-Induced type 2 diabetic experimental rats: a mechanistic approach. Nutrients, 14(19):4181.
[17]. Selvaraj, J., Veeraraghavan, V., Periyasamy, V., and Rajagopal, P., 2021., In Silico and in Vitro Study on the Inhibition of FtsZ Protein of Staphylococcus Aureus by Active Compounds from Andrographis Paniculata. Journal of Biologically Active Products from Nature, 11(2): 116–128. doi:10.1080/22311866.2021.1908163.
[18]. Ponnulakshmi, R., Shyamaladevi, B., Vijayalakshmi, P., & Selvaraj, J., 2019, In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicology Mechanisms and Methods, 29(4): 276–290. https://doi.org/10.1080/15376516.2018.1545815.
[19]. Babu, S., Jayaraman, S., 2020, An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed Pharmacother, 131:110702. doi: 10.1016/j.biopha.2020.110702. Epub 2020 Aug 31. PMID: 32882583.
[20]. Guo, J., Gan, C., Cheng, B., Cui, B., Yi, F., 2023, Exploration of binding mechanism of apigenin to pepsin: Spectroscopic analysis, molecular docking, enzyme activity and antioxidant assays. pectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 290:122281. doi:10.1016/j.saa.2022.122281.
Viewed PDF 210 4 -
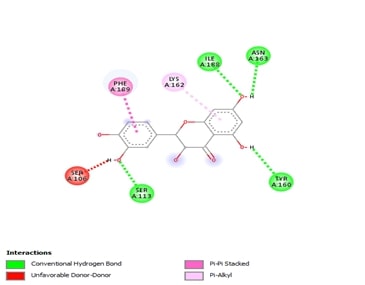 Molecular Docking, Drug-Likeness and Toxicity Prediction to Determine the Role of the Taxifolin on Neurological DiseasesAuthor: Kaviyarasi RenuDOI: 10.21522/TIJPH.2013.SE.25.01.Art009
Molecular Docking, Drug-Likeness and Toxicity Prediction to Determine the Role of the Taxifolin on Neurological DiseasesAuthor: Kaviyarasi RenuDOI: 10.21522/TIJPH.2013.SE.25.01.Art009Molecular Docking, Drug-Likeness and Toxicity Prediction to Determine the Role of the Taxifolin on Neurological Diseases
Abstract:
Through an in-silico approach, the effects of taxifolin on tau, alpha 2 macroglobulin (A2M) and alpha 1 anti-chymotrypsin (ACT), proteins are investigated in relation to neurological disorders. In order to facilitate protein interaction, the ligand taxifolin (extracted from the PubChem Database) and protein receptor molecules (extracted from the PDB) are prepared, converted to PDBQT format, and uploaded in an auto dock. The effects of taxifolin on tau protein, ACT, and A2M are still being studied, but the preliminary findings are promising. These interactions suggest that taxifolin may have a complex role in controlling neuroinflammation, proteostasis, and neurodegeneration in neurological illnesses due to their substantial binding affinity for tau, ACT, and A2M protein. Taxifolin shows promise as a therapy for neurological disorders by targeting tau protein, ACT, and A2M. Lipinski's Rule states that Taxifolin administered orally should not violate more than one condition. Taxifolin examined was in category IV, which is under the dosage of 300 < LD50 = 2000 mg/kg. A significant root mean square value was 0.000, with a docking score of -7.2 for alpha 1 antichymotrypsin and taxifolin. Interactions in 2D and 3D include conventional hydrogen bonds, carbon-hydrogen bonds, and unfavourable donor-donor interactions. The chosen root mean square value was associated with a significant docking score of -10.2 for alpha2 macroglobulin and taxifolin. The docking score for alpha 2 macroglobulin in a two-dimensional structure, emphasising pi-donor bonds, unfavourable donor-donor interactions, and conventional carbon-hydrogen connections. According to these findings, taxifolin has a significant affinity for alpha 2 macroglobulin. Tau had a high root mean square value and a docking score of -7.5 with tau protein. Tau's 2D and 3D structures contain pi-alkyl contacts, pi-stacking interactions, unfavourable donor-donor interactions, and carbon-hydrogen bonds. Its ability to raise A2M activity, decrease ACT expression, and stop tau protein aggregation suggests a variety of potential neuroprotective benefits.
Molecular Docking, Drug-Likeness and Toxicity Prediction to Determine the Role of the Taxifolin on Neurological Diseases
References:
[1]. Topal, F., Nar, M., Gocer, H., Kalin, P., Kocyigit, U. M., Gülçin, İ., & Alwasel, S. H., 2016, Antioxidant Activity of Taxifolin: an Activity–Structure Relationship. Journal of Enzyme Inhibition and Medicinal Chemistry, 31(4), 674-683, http://dx.doi.org/10.3109/14756366.2015.1057723.
[2]. Harborne, J. B., and Williams, C. A., 2000, Advances in Flavonoid Research Since 1992. Phytochemistry, 55(6), 481-504, http://dx.doi.org/10.1016/s0031-9422(00)00235-1.
[3]. Parameswari, R. P., and Lakshmi, T., 2022, Microalgae as a Potential Therapeutic Drug Candidate for Neurodegenerative Diseases. Journal of Biotechnology, 358, 128-139. http://dx.doi.org/10.1016/j.jbiotec.2022.09.009.
[4]. Kumar, S., and Pandey, A. K., 2013, Chemistry and Biological Activities of Flavonoids: An Overview. The Scientific World Journal, 2013(1), p.162750, http://dx.doi.org/10.1155/2013/162750.
[5]. Zhang, C., Zhan, J., Zhao, M., Dai, H., Deng, Y., Zhou, W., and Zhao, L., 2019, Protective Mechanism of Taxifolin for Chlorpyrifos Neurotoxicity in BV2 Cells. Neurotoxicology, 74, 74-80, http://dx.doi.org/10.1016/j.neuro.2019.05.010.
[6]. Sato, M., Murakami, K., Uno, M., Ikubo, H., Nakagawa, Y., Katayama, S., Akagi, K. I., and Irie, K., 2013, Structure-activity Relationship for (+)-Taxifolin Isolated from Silymarin as an Inhibitor of Amyloid β Aggregation. Bioscience, Biotechnology, and Biochemistry, 77(5), 1100-1103, https://academic.oup.com/bbb/article-pdf/77/5/1100/35045235/bbb1100.pdf.
[7]. Sato, M., Murakami, K., Uno, M., Nakagawa, Y., Katayama, S., Akagi, K. I., Masuda, Y., Takegoshi, K., and Irie, K., 2013, Site-specific Inhibitory mechanism for Amyloid β42 Aggregation by Catechol-type Flavonoids Targeting the Lys Residues. Journal of Biological Chemistry, 288(32), 23212-23224, http://dx.doi.org/10.1074/jbc.M113.464222.
[8]. Inoue, T., Saito, S., Tanaka, M., Yamakage, H., Kusakabe, T., Shimatsu, A., Ihara, M., and Satoh-Asahara, N., 2019, Pleiotropic Neuroprotective Effects of Taxifolin in Cerebral Amyloid Angiopathy. Proceedings of the National Academy of Sciences, 116(20), 10031-10038, http://dx.doi.org/10.1073/pnas.1901659116.
[9]. Licastro, F., Mallory, M., Hansen, L. A., and Masliah, E., 1998, Increased levels of α-1-Antichymotrypsin in Brains of Patients with Alzheimer's Disease Correlate with Activated Astrocytes and are Affected by APOE 4 Genotype. Journal of Neuroimmunology, 88(1-2), 105-110, http://dx.doi.org/10.1016/S0165-5728(98)00096-4.
[10]. Abraham, C. R., Selkoe, D. J., and Potter, H., 1988, Immunochemical Identification of the Serine Protease Inhibitor α1-Antichymotrypsin in the Brain Amyloid Deposits of Alzheimer's disease. Cell, 52(4), 487-501, http://dx.doi.org/10.1016/0092-8674(88)90462-X.
[11]. Tyagi, E., Fiorelli, T., Norden, M., and Padmanabhan, J., 2013, Alpha 1‐Antichymotrypsin, an Inflammatory Protein Overexpressed in the Brains of Patients with Alzheimer’s Disease, Induces Tau Hyperphosphorylation through c‐Jun N‐Terminal Kinase Activation. International Journal of Alzheimer’s Disease, 2013(1), p.606083, https://doi.org/10.1155/2013/606083.
[12]. Souparnika. V., Karthik G. M., and Vidya. S., 2023, Antioxidant Activity Of L - Theanine On Cadmium Induced Oxidative Stress Mediated Neurodegeneration - An in vivo Analysis. Journal of Population Therapeutics and Clinical Pharmacology, 29(02), pp. 123–130, https://doi.org/10.47750/jptcp.2022.952.
[13]. Veeraraghavan, V. P., Jayaraman, S., Rengasamy, G., Mony, U., Ganapathy, D. M., Geetha, R. V., and Sekar, D., 2021, Deciphering the role of microRNAs in neuroblastoma. Molecules, 27(1), p.99, http://dx.doi.org/10.3390/molecules27010099.
[14]. Goedert, M., 2004, Tau protein and Neurodegeneration. Seminars in cell & developmental biology, 15(1), 45–9, http://dx.doi.org/10.1016/j.semcdb.2003.12.015.
[16]. Ashraf, G. M., and Alexiou, A., 2019, Biological, Diagnostic and Therapeutic Advances in Alzheimer's Disease. Springer Nature, 293 p, https://play.google.com/store/books/details?id=N8m1DwAAQBAJ.
[17]. Hirshasri, A.G., Renu, K., Priya, V.V., Gayathri, R. and Kavitha, S., The Effect of Aspalathin on SMAD2, SMAD3, TGF-β-A Major Contributor of Inflammation–An In-silico Approach.
[18]. Roy, A., Cheriyan, B.V., Perumal, E., Rengasamy, K.R. and Anandakumar, S., 2024. Effect of hinokitiol in ameliorating oral cancer: in vitro and in silico evidences. Odontology, pp.1-14.
[19]. Saini, R.S., Binduhayyim, R.I.H., Gurumurthy, V., Alshadidi, A.A.F., Aldosari, L.I.N., Okshah, A., Kuruniyan, M.S., Dermawan, D., Avetisyan, A., Mosaddad, S.A. and Heboyan, A., 2024. Dental biomaterials redefined: molecular docking and dynamics-driven dental resin composite optimization. BMC Oral Health, 24(1), p.557.
[20]. Priya, V., Keerthivasan, S. and Kaviyarasi, R., Determining the Dual Effect of Mirabegron on Anticancer Mechanism and Brown Adipose Tissue Activation-An in-silico Approach.
[21]. Neelam, S., Gokara, M., Sudhamalla, B., Amooru, D. G. and Subramanyam, R., 2010, Interaction Studies of Coumaroyltyramine with Human Serum Albumin and its Biological Importance. The Journal of Physical Chemistry B, 114(8), pp.3005-3012, https://pubs.acs.org/doi/abs/10.1021/jp910156k.
[22]. Saini, R.S., Vaddamanu, S.K., Dermawan, D., Bavabeedu, S.S., Khudaverdyan, M., Mosaddad, S.A. and Heboyan, A., 2024. In Silico Docking of Medicinal Herbs Against P. gingivalis for Chronic Periodontitis Intervention. International Dental Journal.
[23]. Alfadda, A. A., Benabdelkamel, H., Masood, A., Jammah, A. A. and Ekhzaimy, A. A., 2018, Differences in the plasma proteome of patients with hypothyroidism before and after thyroid hormone replacement: A proteomic analysis. International Journal of Molecular Sciences, 19(1), p.88, https://www.mdpi.com/1422-0067/19/1/88.
[24]. Yang, J., Zhi, W., and Wang, L., 2024, Role of Tau Protein in Neurodegenerative Diseases and Development of Its Targeted Drugs: A Literature Review. Molecules, 29(12), p.2812, https://www.mdpi.com/1420-3049/29/12/2812.
Viewed PDF 231 11 -
 Antimicrobial Activity of Novel Triterpenoid Derivatives Isolated from Ethyl Acetate Extract of Cassia fistula Stem Bark: In-vitro and In-silico AnalysisAuthor: Rajalakshmi ManikkamDOI: 10.21522/TIJPH.2013.SE.25.01.Art010
Antimicrobial Activity of Novel Triterpenoid Derivatives Isolated from Ethyl Acetate Extract of Cassia fistula Stem Bark: In-vitro and In-silico AnalysisAuthor: Rajalakshmi ManikkamDOI: 10.21522/TIJPH.2013.SE.25.01.Art010Antimicrobial Activity of Novel Triterpenoid Derivatives Isolated from Ethyl Acetate Extract of Cassia fistula Stem Bark: In-vitro and In-silico Analysis
Abstract:
Standard treatments for bacterial infections are becoming increasingly ineffective as antibiotic resistance grows worldwide. Due to the overuse of antibiotics, multidrug-resistant bacteria have emerged as a serious hazard and a major worldwide healthcare issue in the twenty-first century. Traditional approaches to creating novel antibacterial medications are insufficient to fulfil the existing pipeline, hence new tactics in the field of antibacterial discovery are being developed. Cassia fistula (C.fistula), a member of the Leguminosae family, naturally contains antibacterial properties. The plant is used to cure skin diseases, liver problems, tuberculose glands, and hematemesis, pruritus, leucoderma, and diabetes. As a result, effective antimicrobial treatment beyond antibiotics is critical. The Plants contain a wide range of secondary metabolites, including tannins, terpenoids, alkaloids, flavonoids, and glycosides, which have antibacterial characteristics. Terpenenes and terpenoids are effective against bacteria, fungus, viruses, and protozoa. Terpenes' mode of action involves lipophilic chemicals disrupting membranes. Adding a methyl group to increase the hydrophilicity of kaurene diterpenoids decreased their antibacterial efficacy significantly. In the study, antibacterial screening assay against S.aureus and K.pneumonia, a new chemical isolated from C.fistula's ethyl acetate extract demonstrated wider inhibitory zones than the positive control. The treated culture's genomic DNA profile remains unchanged after treatment with the new chemical. The new chemical suppressed protein synthesis, resulting in reduced protein content in treated cultures of both strains, confirming its bactericidal effect. Further immune-blot analysis is required to confirm the particular protein. Investigating a novel triterpenoid that reduces pharmaceutical drug load and resistance risk, as well as treatment costs, could offer promising therapeutic options for treating secondary urinary tract infections associated with diabetes.
Antimicrobial Activity of Novel Triterpenoid Derivatives Isolated from Ethyl Acetate Extract of Cassia fistula Stem Bark: In-vitro and In-silico Analysis
References:
[1]. Deshmukh, R. K., & Gaikwad, K. K., 2022, Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Conversion and Biorefinery, 1-22. doi:10.1007/s13399-022-02623.
[2]. Towers, G. H., Lopez, A., Hudson, J. B, 2001, Antiviral and antimicrobial activities of medicinal plants Journal of Ethnopharmacology, 77, 189-96.https://doi.org/ 10.1016/s0378-8741(01)00292-6.
[3]. Dahanukar, S. A., Kulkarni, R. A, & Rege, N. N.,2000, Pharmacology of medicinal plants and natural products. Indian Journal of Pharmacology, 32(4): S81-S118.
[4]. Rajalakshmi, M., Eliza, J., Cecilia Edel Priya, L. V., et al., 2009, Biochemical and histopathological study of Cassia fistula bark extracts in STZ induced diabetic rats. International Journal of Biotechnology, 2: 134-139.
[5]. Sabapathy, I., Christopher, I., Periyasamy, V., & Manikkam, R., 2022, Molecular docking analysis of tetracyclic triterpenoids from Cassia fistula L. with targets for diabetes mellitus. Bioinformation, 2022, 18(3), 200-205. doi:10.6026/97320630018200.
[6]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P., & Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), 1007-1013.
[7]. Dinga E., Mthiyane, D. M. N., Marume, U, et al., 2022, Biosynthesis of ZnO nanoparticles using Melia azedarach seed extract: evaluation of the cytotoxic and antimicrobial potency. OpenNano, 8,100068.
[8]. Ecevit, K., Barros, A. A., Silva, J. M., & Reis, R. L, 2022, Preventing microbial infections with natural phenolic compounds. Future Pharmacology, 2(4), 460-498.
[9]. Terefe, E. M., 2022, In vitro and in silico pharmacologic evaluation of the antiretroviral activity of croton species. Diss. University of Nairobi.
[10]. Alam, M., Bano, N., Ahmad, T., et al., 2022, Synergistic Role of Plant Extracts and Essential Oils against Multidrug Resistance and Gram-Negative Bacterial Strains Producing Extended-Spectrum β-Lactamases. Antibiotics. 11(7), 855.https://doi.org/10.3390/antibiotics11070855.
[11]. Sruthi, M. A., Mani, G., Ramakrishnan, M., & Selvaraj, J., 2023, Dental caries as a source of Helicobacter pylori infection in children: An RT‐PCR study. International Journal of Paediatric Dentistry, 33(1), 82-88.
[12]. Hochma, E., Yarmolinsky, L., Khalfin, B., et al. 2021, Antimicrobial effect of phytochemicals from edible plants. Processes, 9(11), 2089.
[13]. Pauli, A., Amicbase., 2006, Essential Oils Supplementary Information. Review Science, 13.
[14]. Singh, R., & Sankar, C., 2012, Screening of the Cassia fistula flowers extract for the Anti-acne activity. Res. J. Recent Sci. 2012, 1(11): 53-55.
[15]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P., & Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), 704-713.
[16]. Awal, M. A., Ahsan, S. M., Haque, E., et al., 2010, In vitro antibacterial activity of leaf and root extract of Cassia Fistula. Dinajpur Medical College Journal, 3(1),10-13.
[17]. Sharma, A., Chandraker, S., Patel, V. K., & Ramteke, P., Antibacterial activity of medicinal plants against pathogens causing complicated Urinary Tract Infections. Indian J Pharm Sci. 2009, 71(2): 136-139.https://doi.org/10.4103/0250-474x.54279.
[18]. Webber, D. M., Wallace, M. A., Burnham, C. D., 2022, Stop Waiting for Tomorrow: Disk Diffusion Performed on Early Growth Is an Accurate Method for Antimicrobial Susceptibility Testing with Reduced Turnaround Time. Journal of Clinical Microbiology, 2022, 60(5): e0300720. doi: 10.1128/JCM.03007-20.
[19]. Peterson, L. R., & Shanholtzer, C. J., 1992, Tests for bactericidal effects of antimicrobial agents: technical performance and clinical relevance. Clinical Microbiology Reviews, 5(4), 420-432.https://doi.org/10.1128/cmr.5.4.420
[20]. Spring, K. R., & Davidson, M. W., Introduction to fluorescence microscopy. Nikon MicroscopyU, 09- 28.
[21]. Michele, D. E., Barresi, R., Kanagawa, M., et al., 2002, Post-translational disruption of dystroglycan–ligand interactions in congenital muscular dystrophies. Nature, 418(6896), 417-421.https://doi.org/10.1038/nature00837.
[22]. Ahmad, F., Jabeen, K., Iqbal, S., Umar, A., Ameen, F., Gancarz, M., & Eldin Darwish, D.B., Influence of silicon nanoparticles on Avena sativa L. to alleviate the biotic stress of Rhizoctonia solani. Scientific Reports, 3(1), 15191. doi: 10.1038/s41598-023-41699-w.
[23]. Ahmed, O. M., Saleh, A. S., Ahmed, E. A., Ghoneim, M. M., Ebrahim, H. A., Abdelgawad, M. A., Abdel-Gabbar, M., Efficiency of Bone Marrow-Derived Mesenchymal Stem Cells and Hesperetin in the Treatment of Streptozotocin-Induced Type 1 Diabetes in Wistar Rats. 16(6), 859. doi: 10.3390/ph16060859.
[24]. Krishnan, R. P., Pandiar, D., Ramani, P., & Jayaraman, S., 2025, Molecular profiling of oral epithelial dysplasia and oral squamous cell carcinoma using next generation sequencing. Journal of Stomatology, Oral and Maxillofacial Surgery, 126(4), 102120.
[25]. Mahesh, B., & Satish, S., 2008, Antimicrobial activity of someimportant medicinal plant aga inst plant and humanpathogens. World Journal of Agricultural Sciences, 4, 839-843.
[26]. Babu, P., Dinesh., & Subhasree, R. S., 2009, Antimicrobial activities of Lawsonia inermis-a review. Academic Journal of Plant Sciences, 2(4), 231-232.
[27]. Sher, A., 2009, Antimicrobial activity of natural products from medicinal plants. Gomal Journal of Medical Sciences.7(1), 72-78.
[28]. Pazhani, J., Chanthu, K., Jayaraman, S., & Varun, B. R., 2023, Evaluation of salivary MMP-9 in oral squamous cell carcinoma and oral leukoplakia using ELISA. Journal of Oral and Maxillofacial Pathology, 27(4), 649-654.
[29]. Khatoon, L., Baliraine, F. N., Bonizzoni, M, et al., 2009, Prevalence of antimalarial drug resistance mutations in Plasmodium vivax and P. falciparum from a malaria-endemic area of Pakistan. The American Journal of Tropical Medicine and Hygiene, 81 (3),525.
[30]. El-Jakee, J. K., Nagwa, S., Atta, A., et al., 2011, Antimicrobial resistance in clinical isolates of Staphylococcus aureus from bovine and human sources in Egypt." Glob. Vet. 7, 581-586.
[31]. Patel, P. G., Raval, P. N., et al., 2012, Bacteriological profile and antibiogram of gram-negative organisms isolated from medical and neurology intensive care unit with special reference to multi-drug resistant organisms. National journal of Medical Research, 2 (3), 335-338.
[32]. Fathima, J. S., Jayaraman, S., Sekar, R., & Syed, N. H., 2024, The role of MicroRNAs in the diagnosis and treatment of oral premalignant disorders. Odontology, 1-10. Patel, B.V.,
[33]. Akiyama, H., Fujii, K., Yamasaki, O., et al., 2001, Antibacterial action of several tannins against Staphylococcus aureus. Journal of Antimicrobial Chemotherapy, 48(4), 487-491.
[34]. Ceylan, E., & Daniel, Y. C.,2004, Fung Antimicrobial activity of spices 1. Journal of Rapid Methods & Automation in Microbiology, 12 (1), 1-55. https://doi.org/10.1111/j.1745-4581.2004.tb00046.x.
[35]. Fernandez-Lopez, J., Zhi, N., Aleson-Carbonell, L., et al., 2005, Antioxidant and antibacterial activities of natural extracts: application in beef meatballs." Meat Science, 69, (3), 371-380. https://doi.org/10.1016/j.meatsci.2004.08.004.
[36]. Karsha, P. V., & Lakshmi, O. B., 2010, Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. 2010.
[37]. Yasothkumar, D., Ramani, P., Jayaraman, S., Ramalingam, K., & Tilakaratne, W. M., 2024, Expression Profile of Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck Pathology, 18(1), 28.
[38]. Li, X. Z., Plésiat, P., & Nikaido, H., 2015, The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria." Clinical Microbiology Review, 28, (2), 337-418. https://doi.org/10.1128/CMR.00117-14.
[39]. Mehta, R., & Champney, W. S., 2003, Neomycin and paromomycin inhibit 30S ribosomalsubunit assembly in Staphylococcus aureus. Current Microbiology, 47, 237-243. DOI: 10.1007/s00284-002-3945-9.
[40]. Böddeker, N., Bahador, G., Gibbs, C., et al., 2002, Characterization of a novel antibacterial agentthat inhibits bacterial translation. Cambridge Journal, 8, 1120-1128. 10.1017/s1355838202024020.
[41]. Sagar, S., Ramani, P., Moses, S., Gheena, S., & Selvaraj, J., 2024, Correlation of salivary cytokine IL-17A and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology, 1-10.
Viewed PDF 223 8 -
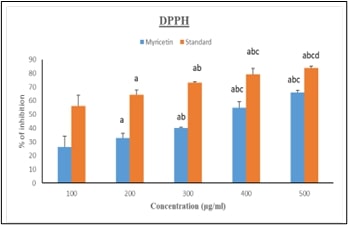 Myricetin Anti-diabetic Activity in 3T3 Cells Targeting the Nrf2-Keap Signaling CascadeAuthor: Jayaraman SDOI: 10.21522/TIJPH.2013.SE.25.01.Art011
Myricetin Anti-diabetic Activity in 3T3 Cells Targeting the Nrf2-Keap Signaling CascadeAuthor: Jayaraman SDOI: 10.21522/TIJPH.2013.SE.25.01.Art011Myricetin Anti-diabetic Activity in 3T3 Cells Targeting the Nrf2-Keap Signaling Cascade
Abstract:
The study was aimed at assessing the effects of Myricetin, a potent anti-cancer compound, targets the NRF2-Keap1 pathway in 3T3-L1 fibroblast cells, which is crucial in cancer progression, cell growth, and metastasis. Various assays have demonstrated myricetin's therapeutic potential. Antioxidant properties, confirmed by the DPPH assay, show dose-dependent free radical inhibition, with myricetin achieving 69.98% inhibition at 500 μg/ml (p<0.001). Anti-inflammatory assays reveal significant reductions in inflammatory markers, with inhibition rising to 74.75% at the same concentration (p<0.001). Gene expression studies highlight myricetin's impact on key components of the NRF2/Keap1 pathway, essential for cancer cell survival. In 3T3-L1 cells treated with myricetin, notable changes were observed in mRNA expression levels: IR (0.95±0.05, p<0.001), IL-1β (0.96±0.04, p<0.002), Keap1 (0.9±0.04, p<0.001), Glut4 (0.6±0.04, p<0.002), NRF2 (0.96±0.4, p<0.001), and NFκB (0.94±0.05, p<0.001). These findings suggest myricetin disrupts critical pathways, contributing to reduced inflammation and potential cancer inhibition. The MTT assay further indicates no cytotoxicity after 48 hours, supporting its safety profile. Molecular docking studies reveal strong binding affinities of myricetin to key pathway components, with Keap1 showing the highest affinity (-9.7 kcal/mol), followed by IR (-8 kcal/mol), NFκB (-6.9 kcal/mol), and NRF2 (-6.9 kcal/mol). IL-1β and Glut4 showed affinities of -7.1 and -7.2 kcal/mol, respectively, reinforcing myricetin's role in modulating the NRF2/Keap1 pathway. In summary, myricetin’s antioxidant, anti-inflammatory, and gene-modulatory activities, combined with its strong molecular interactions, position it as a promising therapeutic agent for both cancer and diabetes by modulating key cellular pathways.
Myricetin Anti-diabetic Activity in 3T3 Cells Targeting the Nrf2-Keap Signaling Cascade
References:
[1]. Narasimhan, A., Sampath, S., Jayaraman, S., & Karundevi, B., 2013, Estradiol favors glucose oxidation in gastrocnemius muscle through modulation of insulin signaling molecules in adult female rats. Endocrine Research, 38(4), 251-262.
[2]. Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Veeraraghavan, V. P., 2022, Effect of Carica papaya on IRS-1/Akt signaling mechanisms in high-fat-diet-streptozotocin-induced type 2 diabetic experimental rats: A mechanistic approach. Nutrients, 14(19), 4181.
[3]. Perumal, S., Langeshwaran, K., Selvaraj, J., Ponnulakshmi, R., Shyamaladevi, B., & Balasubramanian, M. P., 2018, Effect of diosmin on apoptotic signaling molecules in N-nitrosodiethylamine-induced hepatocellular carcinoma in experimental rats. Molecular and Cellular Biochemistry, 449, 27-37.
[4]. Devarajan, N., Jayaraman, S., Mahendra, J., Venkatratnam, P., Rajagopal, P., Palaniappan, H., & Ganesan, S. K., 2021, Berberine—A potent chemosensitizer and chemoprotector to conventional cancer therapies. Phytotherapy Research, 35(6), 3059-3077.
[5]. Jayaraman, S., Natararaj, S., & Veeraraghavan, V. P., 2024, Hesperidin inhibits oral cancer cell growth via apoptosis and inflammatory signaling-mediated mechanisms: Evidence from in vitro and in silico analyses. Cureus, 16(2).
[6]. Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Veeraraghavan, V. P., 2022, Carica papaya reduces muscle insulin resistance via IR/GLUT4 mediated signaling mechanisms in high fat diet and streptozotocin-induced type-2 diabetic rats. Antioxidants, 11(10), 2081.
[7]. Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Prasad, M., 2023, Carica papaya reduces high fat diet and streptozotocin-induced development of inflammation in adipocyte via IL-1β/IL-6/TNF-α mediated signaling mechanisms in type-2 diabetic rats. Current Issues in Molecular Biology, 45(2), 852.
[8]. Indu, S., Vijayalakshmi, P., Selvaraj, J., & Rajalakshmi, M., 2021, Novel triterpenoids from Cassia fistula stem bark depreciates STZ-induced detrimental changes in IRS-1/Akt-mediated insulin signaling mechanisms in type-1 diabetic rats. Molecules, 26(22), 6812.
[9]. Gupta, G., Siddiqui, M. A., Khan, M. M., Ajmal, M., Ahsan, R., Rahaman, M. A., & Khushtar, M., 2020, Current pharmacological trends on myricetin. Drug Research, 70(10), 448-454.
[10]. Semwal, D. K., Semwal, R. B., Combrinck, S., & Viljoen, A., 2016, Myricetin: A dietary molecule with diverse biological activities. Nutrients, 8(2), 90.
[11]. Afroze, N., Pramodh, S., Hussain, A., Waleed, M., & Vakharia, K., 2020, A review on myricetin as a potential therapeutic candidate for cancer prevention. 3 Biotech, 10(5), 211.
[12]. Hu, Y., Jiang, Q., Zhai, X., Liu, L., & Hong, Y., 2023, Screening and validation of the optimal panel of reference genes in colonic epithelium and relative cancer cell lines. Scientific Reports, 13(1), 17777.
[13]. Bhat, I. A., Naykoo, N. A., Qasim, I., Ganie, F. A., Yousuf, Q., Bhat, B. A., Rasool, R., Aziz, S. A., & Shah, Z. A., 2014, Association of interleukin 1 beta (IL-1β) polymorphism with mRNA expression and risk of non-small cell lung cancer. Meta gene, 2, 123–133.
[14]. Singh, A., Misra, V., Thimmulappa, R. K., Lee, H., Ames, S., Hoque, M. O., Herman, J. G., Baylin, S. B., Sidransky, D., Gabrielson, E., Brock, M. V., & Biswal, S., 2006, Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Medicine, 3(10), e420.
[15]. Xiong, L., Xie, J., Song, C., Liu, J., Zheng, J., Liu, C., Zhang, X., Li, P., & Wang, F., 2015, The Activation of Nrf2 and Its Downstream Regulated Genes Mediates the Antioxidative Activities of Xueshuan Xinmaining Tablet in Human Umbilical Vein Endothelial Cells. Evidence-based complementary and alternative medicine: eCAM, 2015, 187265.
[16]. Neralla, M., Preethi, A., Selvakumar, S. C., & Sekar, D., 2023, Expression levels of microRNA-7110 in oral squamous cell carcinoma. Minerva Dental and Oral Science, 73(3), 155–160.
[17]. Lalit, H., 2023, Preparation, Characterization, and Evaluation of Cytotoxic Activity of a Novel Titanium Dioxide Nanoparticle-infiltrated Orthodontic Adhesive: An In Vitro Study. World Journal of Dentistry, 14(10), 882-887.
[18]. Smith, S. M., Lyu, Y. L., & Cai, L., 2014, NF-κB affects proliferation and invasiveness of breast cancer cells by regulating CD44 expression. PloS One, 9(9), e106966.
[19]. Arponen, M., Jalava, N., Widjaja, N., & Ivaska, K. K., 2022, Glucose transporters GLUT1, GLUT3, and GLUT4 have different effects on osteoblast proliferation and metabolism. Frontiers in Physiology, 13, 1035516.
[20]. Bae, T., Hallis, S. P., & Kwak, M. K., 2024, Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Experimental & Molecular Medicine, 56(3), 501–514. https://doi.org/10.1038/s12276-024-01180-8.
[21]. Uruno, A., Yagishita, Y., & Yamamoto, M., 2015, The Keap1-Nrf2 system and diabetes mellitus. Archives of Biochemistry and Biophysics, 566, 76–84. https://doi.org/10.1016/j.abb.2014.12.012.
[22]. An, Y., Xu, B. T., Wan, S. R., Ma, X. M., Long, Y., Xu, Y., & Jiang, Z. Z., 2023, The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovascular Diabetology, 22(1), 237. https://doi.org/10.1186/s12933-023-01965-7.
[23]. Gopalakrishnan, U., Murthy, R. T., Felicita, A. S., Alshehri, A., Awadh, W., Almalki, A., & Patil, S., 2023, Sulfate-reducing bacteria in patients undergoing fixed orthodontic treatment. International Dental Journal, 73(2), 274-279.
Viewed PDF 223 6 -
 Glycemic Gums: Unveiling the Phytochemical Connection Between Diabetes and Oral Health: A ReviewAuthor: Sridevi GopathyDOI: 10.21522/TIJPH.2013.SE.25.01.Art012
Glycemic Gums: Unveiling the Phytochemical Connection Between Diabetes and Oral Health: A ReviewAuthor: Sridevi GopathyDOI: 10.21522/TIJPH.2013.SE.25.01.Art012Glycemic Gums: Unveiling the Phytochemical Connection Between Diabetes and Oral Health: A Review
Abstract:
Hyperglycemia, a pathological condition, predominantly defines diabetes mellitus, a persistent and complex metabolic dysfunction. It has a significant impact on oral health, as seen by conditions including periodontal disease, xerostomia (dry mouth), and increased susceptibility to infections. The intricate bidirectional relationship that exists between oral health and the metabolic disorder diabetes has been thoroughly documented, where it has been observed with poor oral hygiene worsening glycemic management and vice versa. Recently, there has been a change in focus toward the utilization of phytochemicals, the bioactive substances found in plants, as an additional treatment strategy for addressing issues related to dental health and diabetes. Phytochemicals exhibit antimicrobial, anti-inflammatory, and antioxidant characteristics that are critical for battling oral infections as well as reducing the inflammatory processes associated with diabetic periodontitis. Evidence shows that these substances contribute to enhanced insulin sensitivity and glycemic management, in addition to their beneficial effects on dental health, such as suppressing inflammation and oxidative stress. This review investigates some complex interactions between diabetes and oral hygiene, as well as the role of inflammatory mediators, cellular oxidative stress, and dysfunction of the salivary gland. It explores the dual role of phytochemicals such as alkaloids, flavonoids, tannins, reseveratol, and saponins in the context of oral health care for diabetic individuals.
Glycemic Gums: Unveiling the Phytochemical Connection Between Diabetes and Oral Health: A Review
References:
[1]. Lecube, A., 2024, Impact of obesity and diabetes on health and cardiovascular disease. Atencion Primaria, 56(12), 103045-103045.
[2]. Mekala, K. C., & Bertoni, A. G., 2020, Epidemiology of diabetes mellitus. In Transplantation, bioengineering, and regeneration of the endocrine pancreas, pp. 49-58, Academic Press.
[3]. Nayee, S., Ormond, M., Sanderson, J. D., & Escudier, M. P., 2024, Disorders of the mouth. Medicine.
[4]. Yasothkumar, D., Ramani, P., Jayaraman, S., Ramalingam, K., & Tilakaratne, W. M., 2024, Expression Profile of Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck Pathology, 18(1), 28.
[5]. Stoopler, E. T., Villa, A., Bindakhil, M., Díaz, D. L. O., & Sollecito, T. P., 2024, Common oral conditions: a review. JAMA, 331(12), 1045-1054.
[6]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P., & Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), 704-713.
[7]. Hessain, D., Dalsgaard, E. M., Norman, K., Sandbæk, A., & Andersen, A., 2023, Oral health and type 2 diabetes in a socioeconomic perspective. Primary Care Diabetes, 17(5), 466-472. https://doi.org/10.1016/j.pcd.2023.07.001
[8]. Krishnan, R. P., Pandiar, D., Ramani, P., & Jayaraman, S., 2025, Molecular profiling of oral epithelial dysplasia and oral squamous cell carcinoma using next generation sequencing. Journal of Stomatology, Oral and Maxillofacial Surgery, 126(4), 102120.
[9]. Pazhani, J., Chanthu, K., Jayaraman, S., & Varun, B. R., 2023, Evaluation of salivary MMP-9 in oral squamous cell carcinoma and oral leukoplakia using ELISA. Journal of Oral and Maxillofacial Pathology, 27(4), 649-654.
[10]. Yucel-Lindberg, T., & Båge, T., 2013, Inflammatory mediators in the pathogenesis of periodontitis. Expert Reviews in Molecular Medicine, 15, e7. https://doi.org/10.1017/erm.2013.8
[11]. Li, S., Li, H., Kong, H., Wu, S. Y., Cheng, C. K., & Xu, J., 2023, Endogenous and microbial biomarkers for periodontitis and type 2 diabetes mellitus. Frontiers in Endocrinology, 14, 1292596. https://doi.org/10.3389/fendo.2023.1292596
[12]. Torrungruang, K., Ongphiphadhanakul, B., Jitpakdeebordin, S., & Sarujikumjornwatana, S., 2018, Mediation analysis of systemic inflammation on the association between periodontitis and glycaemic status. Journal of Clinical Periodontology, 45(5), 548-556. https://doi.org/10.1111/jcpe.12884
[13]. Arshad, R., Ismail, W. A., Zara, B., Naseer, R., Minhas, S., Ansari, M., & Alam, M. K., 2022, Salivary MMP-9 levels in chronic periodontitis patients with type-II diabetes mellitus. Molecules, 27(7), 2174. https://doi.org/10.3390/molecules27072174
[14]. Singh, H., Singh, R., Singh, A., Singh, H., Singh, G., Kaur, S., & Singh, B., 2024, Role of oxidative stress in diabetes-induced complications and their management with antioxidants. Archives of Physiology and Biochemistry, 130(6), 616-641. https://doi.org/10.1080/13813455.2023.2243651
[15]. Tang, L., Li, T., Chang, Y., Wang, Z., Li, Y., Wang, F., & Sui, L., 2022, Diabetic oxidative stress-induced telomere damage aggravates periodontal bone loss in periodontitis. Biochemical and Biophysical Research Communications, 614, 22-28. https://doi.org/10.1016/j.bbrc.2022.04.039
[16]. Pasupuleti, M. K., Nagate, R. R., Alqahtani, S. M., Penmetsa, G. S., Gottumukkala, S. N., & Ramesh, K. S. V., 2023, Role of medicinal herbs in periodontal therapy: a systematic review. Journal of International Society of Preventive and Community Dentistry, 13(1), 9-16. https://doi.org/10.4103/jispcd.jispcd_210_22
[17]. Fathima, J. S., Jayaraman, S., Sekar, R., & Syed, N. H., 2024, The role of MicroRNAs in the diagnosis and treatment of oral premalignant disorders. Odontology, 1-10.
[18]. Sen, D. B., Balaraman, R., Sen, A. K., Zanwar, A. S., Greeshma, K. P., & Maheshwari, R. A., 2023, Anti-diabetic activity of herbal remedies. Journal of Natural Remedies, 373-381. https://doi.org/10.18311/jnr/2023/32182
[19]. Sivakumar, A., Thanu, A. S., Vishnumukkala, T., Ksv, A. B. G., Shetty, J. K., Jagadeesan, S., & Gopalakrishna, P. K., 2024, Management of diabetes mellitus using medicinal plants: A review. Bioinformation, 20(7), 705.https://doi.org/10.6026/973206300200705
[20]. Djannah, F., Rahaju, A. S., Kadriyan, H., Triani, E., Trianto, H. F., & Zainul, R., 2024, Black Garlic for the treatment of Tuberculosis and Diabetes mellitus. Research Journal of Pharmacy and Technology, 17(3), 1282-1288. https://doi.org/10.52711/0974-360X.2024.00201
[21]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P., & Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), 1007-1013.
[22]. Muhammad, I., Rahman, N., Nishan, U., & Shah, M., 2021, Antidiabetic activities of alkaloids isolated from medicinal plants. Brazilian Journal of Pharmaceutical Sciences, 57, e19130. https://doi.org/10.1590/s2175-97902020000419130
[23]. Behl, T., Gupta, A., Albratty, M., Najmi, A., Meraya, A. M., Alhazmi, H. A., & Bungau, S. G., 2022, Alkaloidal phytoconstituents for diabetes management: exploring the unrevealed potential. Molecules, 27(18), 5851.
[24]. Ajebli, M., Khan, H., & Eddouks, M., 2021, Natural alkaloids and diabetes mellitus: A review. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders), 21(1), 111-130. https://doi.org/10.2174/1871530320666200821124817
[25]. Sruthi, M. A., Mani, G., Ramakrishnan, M., & Selvaraj, J., 2023, Dental caries as a source of Helicobacter pylori infection in children: An RT‐PCR study. International Journal of Paediatric Dentistry, 33(1), 82-88.
[27]. Mohammadian Haftcheshmeh, S., & Momtazi‐Borojeni, A. A., 2021, Berberine as a promising natural compound for the treatment of periodontal disease: A focus on anti‐inflammatory properties. Journal of Cellular and Molecular Medicine, 25(24), 11333-11337. https://doi.org/10.1111/jcmm.17019
[28]. Zhang, L. N., Wang, X. X., Wang, Z., Li, K. Y., Xu, B. H., & Zhang, J., 2019, Berberine improves advanced glycation end products-induced osteogenic differentiation responses in human periodontal ligament stem cells through the canonical Wnt/β-catenin pathway. Molecular Medicine Reports, 19(6), 5440-5452. https://doi.org/10.3892/mmr.2019.10193
[29]. Kumar, A., Aswal, S., Semwal, R. B., Chauhan, A., Joshi, S. K., & Semwal, D. K., 2019, Role of plant-derived alkaloids against diabetes and diabetes-related complications: a mechanism-based approach. Phytochemistry Reviews, 18(5), 1277-1298. https://doi.org/10.1007/s11101-019-09648-6
[30]. Huang, L. J., Lan, J. X., Wang, J. H., Huang, H., Lu, K., Zhou, Z. N., & Hou, W., 2024, Bioactivity and mechanism of action of sanguinarine and its derivatives in the past 10 years. Biomedicine & Pharmacotherapy, 173, 116406. https://doi.org/10.1016/j.biopha.2024.116406
[31]. Aljubouri, E. A., & Alaubydi, M. A., 2023, Investigation of the Association of Oral Infections with Diabetes Mellitus. Iraqi Journal of Science, 4427-4435. https://doi.org/10.24996/ijs.2023.64.9.12
[32]. Wahab, A., Batool, F., Muhammad, M., Zaman, W., Mikhlef, R. M., & Naeem, M., 2023, Current knowledge, research progress, and future prospects of phyto-synthesized nanoparticles interactions with food crops under induced drought stress. Sustainability, 15(20), 14792. https://doi.org/10.4018/978-1-4666-9494-1.ch013
[33]. Horvat, A., Vlašić, I., Štefulj, J., Oršolić, N., & Jazvinšćak Jembrek, M., 2023, Flavonols as a Potential Pharmacological Intervention for Alleviating Cognitive Decline in Diabetes: Evidence from Preclinical Studies. Life, 13(12), 2291. https://doi.org/10.3390/life13122291
[34]. Sagar, S., Ramani, P., Moses, S., Gheena, S., & Selvaraj, J., 2024, Correlation of salivary cytokine IL-17A and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology, 1-10.
[35]. Ren, J. I. E., Lu, Y., Qian, Y., Chen, B., Wu, T. A. O., & Ji, G., 2019, Recent progress regarding kaempferol for the treatment of various diseases. Experimental and Therapeutic Medicine, 18(4), 2759-2776. https://doi.org/10.3892/etm.2019.7886
[36]. Shahbaz, M., Imran, M., Momal, U., Naeem, H., Alsagaby, S. A., Al Abdulmonem, W., & Jbawi, E. A., 2023, Potential effect of kaempferol against various malignancies: recent advances and perspectives. Food and Agricultural Immunology, 34(1), 2265690. https://doi.org/10.1080/09540105.2023.2265690
[37]. Homayouni, F., Haidari, F., Hedayati, M., Zakerkish, M., & Ahmadi, K., 2018, Blood pressure lowering and anti‐inflammatory effects of hesperidin in type 2 diabetes; a randomized double‐blind controlled clinical trial. Phytotherapy Research, 32(6), 1073-1079. https://doi.org/10.1002/ptr.6046
[38]. Huang, C. Y., Ng, M. Y., Lin, T., Liao, Y. W., Huang, W. S., Hsieh, C. W., & Chen, C. J., 2024, Quercetin ameliorates advanced glycation end product-induced wound healing impairment and inflammaging in human gingival fibroblasts. Journal of Dental Sciences, 19(1), 268-275. https://doi.org/10.1016/j.jds.2023.04.014
[39]. Mooney, E. C., Holden, S. E., Xia, X. J., Li, Y., Jiang, M., Banson, C. N., & Sahingur, S. E., 2021, Quercetin preserves oral cavity health by mitigating inflammation and microbial dysbiosis. Frontiers in Immunology, 12, 774273. https://doi.org/10.3389/fimmu.2021.774273
[40]. Liu, C., Zhang, S., Bai, H., Zhang, Y., Jiang, Y., Yang, Z., & Ding, Y., 2022, Soy isoflavones alleviate periodontal destruction in ovariectomized rats. Journal of Periodontal Research, 57(3), 519-532. https://doi.org/10.1111/jre.12981
[41]. Mistry, P. S., Chorawala, M. R., Sivamaruthi, B. S., Prajapati, B. G., Kumar, A., & Chaiyasut, C., 2024, The Role of Dietary Anthocyanins for Managing Diabetes Mellitus-Associated Complications. Current Diabetes Reviews. https://doi.org/10.2174/0115733998322754240802063730
[42]. Mao, T., Akshit, F. N. U., & Mohan, M. S., 2023, Effects of anthocyanin supplementation in diet on glycemic and related cardiovascular biomarkers in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Frontiers in Nutrition, 10, 1199815. https://doi.org/10.3389/fnut.2023.1199815
[43]. Al-Janabi, A. A. H. S., 2023, A positive or negative connection of diabetes mellitus to the oral microbiota. The Eurasian journal of medicine, 55(1), 83. https://doi.org/10.5152/eurasianjmed.2023.21164
[44]. Kováč, J., Slobodníková, L., Trajčíková, E., Rendeková, K., Mučaji, P., Sychrová, A., & Bittner Fialová, S., 2022, Therapeutic potential of flavonoids and tannins in management of oral infectious diseases—A review. Molecules, 28(1), 158. https://doi.org/10.3390/molecules28010158
[45]. Su, K., Li, J., Wu, X., Deng, D., Gu, H., Sun, Y., & Wu, K., 2024, One‐Step Synthesis of Hydrogel Adhesive with Acid‐Responsive Tannin Release for Diabetic Oral Mucosa Defects Healing. Advanced Healthcare Materials, 13(9), 2303252. https://doi.org/10.1002/adhm.202303252
[46]. Bhattarai, K. R., Lee, S. W., Kim, S. H., Kim, H. R., & Chae, H. J., 2017, Ixeris dentata extract regulates salivary secretion through the activation of aquaporin-5 and prevents diabetes-induced xerostomia. Journal of experimental pharmacology, 81-91. https://doi.org/10.2147/jep.s141807
[47]. E Akpotu, A., I Ghasi, S., O Ike, A., F Akhigbe, O., A Amadi, M., OJ Ajah, D., & U Ukiwa, M., 2024, Reducing Sugar, Alkaloid and Tannin from Dryopteris dilatata Fractions Modulates Diabetogenic and Oxidative Stress Activity on Alloxan Induced Diabetic Rats. Asian Journal of Research in Medical and Pharmaceutical Sciences, 13(3), 21-33. https://doi.org/10.9734/ajrimps/2024/v13i3259
[48]. Gandhi, G. R., Antony, P. J., Ceasar, S. A., Vasconcelos, A. B. S., Montalvão, M. M., Farias de Franca, M. N., & Gan, R. Y., 2024, Health functions and related molecular mechanisms of ellagitannin-derived urolithins. Critical Reviews in Food Science and Nutrition, 64(2), 280-310. https://doi.org/10.1080/10408398.2022.2106179
[49]. Zare Javid, A., Hormoznejad, R., Yousefimanesh, H. A., Zakerkish, M., Haghighi‐zadeh, M. H., Dehghan, P., & Ravanbakhsh, M., 2017, The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytotherapy Research, 31(1), 108-114. https://doi.org/10.1002/ptr.5737
[50]. Liu, F., Smith, A. D., Wang, T. T., Pham, Q., Yang, H., & Li, R. W., 2023, Ellagitannin punicalagin disrupts the pathways related to bacterial growth and affects multiple pattern recognition receptor signalling by acting as a selective histone deacetylase inhibitor. Journal of Agricultural and Food Chemistry, 71(12), 5016-5026. https://doi.org/10.1021/acs.jafc.2c08738
[51]. Javid, A. Z., Hormoznejad, R., Allah Yousefimanesh, H., Haghighi-Zadeh, M. H., & Zakerkish, M., 2019, Impact of resveratrol supplementation on inflammatory, antioxidant, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 13(4), 2769-2774. https://doi.org/10.1016/j.dsx.2019.07.042
[52]. Tan, Y., Feng, J., Xiao, Y., & Bao, C., 2022, Grafting resveratrol onto mesoporous silica nanoparticles towards efficient sustainable immunoregulation and insulin resistance alleviation for diabetic periodontitis therapy. Journal of Materials Chemistry B, 10(25), 4840-4855. https://doi.org/10.1039/d2tb00484d
[53]. Zare Javid, A., Hormoznejad, R., Yousefimanesh, H. A., Zakerkish, M., Haghighi‐zadeh, M. H., Dehghan, P., & Ravanbakhsh, M., 2017, The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytotherapy Research, 31(1), 108-114. https://doi.org/10.1002/ptr.5737
[54]. Nowak, E., Psiuk, D., Rocka, A., Dycha, N., Jasielski, P., Jasielska, F., & Rocka, E., 2022, Resveratrol impacts health in patients with diabetes mellitus and other metabolic conditions. Journal of Education, Health and Sport, 12(11), 341-346. https://doi.org/10.12775/jehs.2022.12.11.045
[55]. Yu, X., Jia, Y., & Ren, F., 2024, Multidimensional biological activities of resveratrol and its prospects and challenges in the health field. Frontiers in Nutrition, 11, 1408651. https://doi.org/10.3389/fnut.2024.1408651
[56]. Hwang, S. M., Kim, T. Y., Kim, A., Kim, Y. G., Park, J. W., Lee, J. M., & Suh, J. Y., 2024, Resveratrol facilitates bone formation in high-glucose conditions. Frontiers in Physiology, 15, 1347756. https://doi.org/10.3389/fphys.2024.1347756
[57]. Miyashiro, C. A., Bernegossi, J., Bonifácio, B. V., de Toledo, L. G., Ramos, M. A. S., Bauab, T. M., & Chorilli, M., 2020, Development and characterization of a novel liquid crystalline system containing sodium alginate for incorporation of trans-resveratrol intended for treatment of buccal candidiasis. Die Pharmazie-An International Journal of Pharmaceutical Sciences, 75(5), 179-185.
[58]. Reis, F. N., Câmara, J. V. F., Dionizio, A., Araujo, T. T., da Silva, N. D. G., Levy, F. M., & Buzalaf, M. A. R., 2024, Increase in plasma resveratrol levels and in acid-resistant proteins in the acquired enamel pellicle after use of resveratrol-containing orodispersible tablets. Journal of Dentistry, 143, 104876. https://doi.org/10.1016/j.jdent.2024.104876
[59]. Reis, F. N., Câmara, J. V. F., Dionizio, A., Araujo, T. T., da Silva, N. D. G., Levy, F. M., & Buzalaf, M. A. R., 2024, Increase in plasma resveratrol levels and in acid-resistant proteins in the acquired enamel pellicle after use of resveratrol-containing orodispersible tablets. Journal of Dentistry, 143, 104876. https://doi.org/10.22376/ijlpr.2023.13.6.l349-l357
[60]. Pashapour, S., Saberivand, A., Khaki, A. A., & Saberivand, M., 2023, Effect of saponin on spermatogenesis and testicular structure in streptozotocin-induced diabetic mice. In Veterinary Research Forum (Vol. 14, No. 11, p. 601). Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. https://doi.org/10.30466/vrf.2023.1986019.3727
[61]. Tang, P., Liu, S., Zhang, J., Ai, Z., Hu, Y., Cui, L., & Wang, Y., 2024, Ginsenosides as dietary supplements with immunomodulatory effects: a review. Applied Biological Chemistry, 67(1), 27. https://doi.org/10.1186/s13765-024-00881-y
[62]. Chen, J., Ullah, H., Zheng, Z., Gu, X., Su, C., Xiao, L., Wu, X., Xiong, F., Li, Q., & Zha, L., 2020, Soyasaponins reduce inflammation by downregulating MyD88 expression and suppressing the recruitments of TLR4 and MyD88 into lipid rafts. BMC Complementary Medicine and Therapies, 20(1). https://doi.org/10.1186/s12906-020-2864-2
[63]. Kuzu, T. E., Ozturk, K., Gurgan, C. A., Yay, A., Goktepe, O., & Kantarcı, A., 2023, Anti-inflammatory and pro-regenerative effects of a monoterpene glycoside on experimental periodontitis in a rat model of diabetes. Journal of Periodontal Research, 58(5), 932–938. https://doi.org/10.1111/jre.13151
[64]. Pallod, S., Aguilera Olvera, R., Ghosh, D., Rai, L., Brimo, S., DeCambra, W., Sant, H. G., Ristich, E., Singh, V., Abedin, M. R., Chang, N., Yarger, J. L., Lee, J. K., Kilbourne, J., Yaron, J. R., Haydel, S. E., & Rege, K., 2024, Skin repair and infection control in diabetic, obese mice using bioactive laser-activated sealants. Biomaterials, 311, 122668. https://doi.org/10.1016/j.biomaterials.2024.122668
[65]. Antolak, H., Mizerska, U., Berłowska, J., Otlewska, A., & Kręgiel, D., 2018, Quillaja saponaria saponins with potential to enhance the effectiveness of disinfection processes in the beverage industry. Applied Sciences, 8(3), 368. https://doi.org/10.3390/app8030368
Viewed PDF 274 10 -
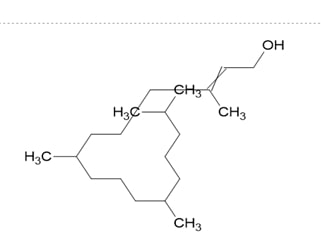 Exploring the Antioxidant Activity of Phytol from the Scoparia dulcis Through In-silico AnalysisAuthor: M. RajalakshmiDOI: 10.21522/TIJPH.2013.SE.25.01.Art013
Exploring the Antioxidant Activity of Phytol from the Scoparia dulcis Through In-silico AnalysisAuthor: M. RajalakshmiDOI: 10.21522/TIJPH.2013.SE.25.01.Art013Exploring the Antioxidant Activity of Phytol from the Scoparia dulcis Through In-silico Analysis
Abstract:
Diabetes is a long-term physiological disorder that affects people of all ages and has a major negative impact on people's ability to live normal, harmonious lives across the globe. The discovery of innovative antidiabetic medications is necessary due to the emergence of resistance and adverse effects of existing oral antidiabetic drugs, even with the availability of insulin preparations and various synthetic alternatives. Due to the side effects, Scientists are focusing on Phytotherapy. This computational study aimed to elucidate the antioxidant activity of Phytol by utilizing molecular docking. The objective of the current research is to predict the Lipinski rule of 5 for Phytol. To examine the ADMET properties of Phytol. To analyse the protein-ligand interaction of Phytol with ROS proteins like Superoxide dismutase (PDB ID:1SPD_A), Catalase (PDB ID:1QQW_A), Glutathione peroxidase (PDB ID:2HE3_A), Peroxiredoxin (PDB ID:1OC3_A). The results of the in-silico studies infer that Phytol follows the Lipinski rule of 5, having a higher binding affinity with catalase protein and better hydrogen bond interactions with Superoxide dismutase and Glutathione peroxidase. The current study provides evidence that Phytol reduces oxidative stress. However, additional in-vitro and in-vivo research are needed to understand the prediction process.
Exploring the Antioxidant Activity of Phytol from the Scoparia dulcis Through In-silico Analysis
References:
[1]. GBD, 2021, Diabetes Collaborators, 2023, Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet (London, England), 402(10397), 203–234. https://doi.org/10.1016/S0140-6736(23)01301-6.
[2]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P., & Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), 1007-1013.
[3]. Kharroubi, A. T., & Darwish, H. M., 2015, Diabetes mellitus: The epidemic of the century. World Journal of Diabetes,6(6),850–867. https://doi.org/10.4239/wjd.v6.i6.850.
[4]. Baynes, J. W., & Thorpe, S. R., 1999, Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes, 48(1), 1–9. https://doi.org/10.2337/diabetes.48.1.1
[5]. Sruthi, M. A., Mani, G., Ramakrishnan, M., & Selvaraj, J., 2023, Dental caries as a source of Helicobacter pylori infection in children: An RT‐PCR study. International Journal of Paediatric Dentistry, 33(1), 82-88.
[6]. Navrot, N., Rouhier, N., Gelhaye, E., & Jacquot, J. P., 2007, Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiologia Plantarum, 129(1), 185-195.
[7]. Mittler, R., Vanderauwera, S., Gollery, M., & Van Breusegem, F., 2004, Reactive oxygen gene network of plants. Trends in Plant Science, 9(10), 490–498. https://doi.org/10.1016/j.tplants.2004.08.009.
[8]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P., & Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), 704-713.
[9]. Li, G. Q., Kam, A., Wong, K. H., Zhou, X., Omar, E. A., Alqahtani, A., Li, K. M., Razmovski-Naumovski, V., & Chan, K., 2012, Herbal medicines for the management of diabetes. Advances in Experimental Medicine and Biology, 771, 396–413. https://doi.org/10.1007/978-1-4614-5441-0_28.
[10]. Kazeem, M. I., & Davies, T. C., 2016, Anti-diabetic functional foods as sources of insulin secreting, insulin sensitizing and insulin mimetic agents. Journal of Functional Foods, 20, 122-138.
[11]. Krishnan, R. P., Pandiar, D., Ramani, P., & Jayaraman, S., 2025, Molecular profiling of oral epithelial dysplasia and oral squamous cell carcinoma using next generation sequencing. Journal of Stomatology, Oral and Maxillofacial Surgery, 126(4), 102120.
[12]. Reshma Rajan, R. R., Mahima Vedi, M. V., Badrinathan Sridharan, B. S., Himaja, M., Sabina, E. P., & Raj, N. A. N., 2014, In vitro and in vivo study on the effect of Scoparia dulcis in inhibiting the growth of urinary crystals. Internatinal Journal of Phytomedicine 6, 617-624.
[13]. Pazhani, J., Chanthu, K., Jayaraman, S., & Varun, B. R., 2023, Evaluation of salivary MMP-9 in oral squamous cell carcinoma and oral leukoplakia using ELISA. Journal of Oral and Maxillofacial Pathology, 27(4), 649-654.
[14]. Brown, P. J., Mei, G., Gibberd, F. B., Burston, D., Mayne, P. D., McClinchy, J. E., & Sidey, M., 1993, Diet and Refsum's disease. The determination of phytanic acid and phytol in certain foods and the application of this knowledge to the choice of suitable convenience foods for patients with Refsum's disease. Journal of Human Nutrition and Dietetics, 6(4), 295-305.
[15]. Islam, M. T., Ali, E. S., Uddin, S. J., Shaw, S., Islam, M. A., Ahmed, M. I., Chandra Shill, M., Karmakar, U. K., Yarla, N. S., Khan, I. N., Billah, M. M., Pieczynska, M. D., Zengin, G., Malainer, C., Nicoletti, F., Gulei, D., Berindan-Neagoe, I., Apostolov, A., Banach, M., Yeung, A. W. K.,Atanasov, A. G., 2018, Phytol: A review of biomedical activities. Food and chemical toxicology: An International Journal Published for the British Industrial Biological Research Association, 121, 82–94. https://doi.org/10.1016/j.fct.2018.08.032.
[16]. Fathima, J. S., Jayaraman, S., Sekar, R., & Syed, N. H., 2024, The role of MicroRNAs in the diagnosis and treatment of oral premalignant disorders. Odontology, 1-10.
[17]. Eliza, J., Daisy, P., & Ignacimuthu, S., 2010, Antioxidant activity of costunolide and eremanthin isolated from Costus speciosus (Koen ex. Retz) Sm. Chemico-Biological Interactions, 188(3), 467–472. https://doi.org/10.1016/j.cbi.2010.08.002.
[18]. Brooijmans, N., & Kuntz, I. D., 2003, Molecular recognition and docking algorithms. Annual Review of Biophysics and Biomolecular Structure, 32, 335–373. https://doi.org/10.1146/annurev.biophys.32.110601.142532.
[19]. Kitchen, D. B., Decornez, H., Furr, J. R., & Bajorath, J., 2004, Docking and scoring in virtual screening for drug discovery: methods and applications. Nature reviews. Drug Discovery, 3(11), 935–949. https://doi.org/10.1038/nrd1549.
[20]. Pari, L., & Latha, M., 2004, Protective role of Scoparia dulcis plant extract on brain antioxidant status and lipidperoxidation in STZ diabetic male Wistar rats. BMC Complementary and Alternative Medicine, 4, 16. https://doi.org/10.1186/1472-6882-4-16.
[21]. Lin, H. D., Lee, Y. C., Chiang, C. Y., Lin, Y. J., Shih, C. Y., Tsai, R. K., Lin, P. Y., Lin, S. Z., Ho, T. J., & Huang, C. Y., 2023, Protective effects of Scoparia dulcis L. extract on high glucose-induced injury in human retinal pigment epithelial cells. Frontiers in Nutrition, 10, 1085248. https://doi.org/10.3389/fnut.2023.1085248.
[22]. Sivasankarapillai, V. S., Krishnamoorthy, N., Eldesoky, G. E., Wabaidur, S. M., Islam, M. A., Dhanusuraman, R., & Ponnusamy, V. K., 2022, One-pot green synthesis of ZnO nanoparticles using Scoparia dulcis plant extract for antimicrobial and antioxidant activities. Applied Nanoscience, 1–11.Advance online publication. https://doi.org/10.1007/s13204-022-02610-7.
[23]. Mishra, M. R., Mishra, A., Pradhan, D. K., Panda, A. K., Behera, R. K., & Jha, S., 2013, Antidiabetic and Antioxidant Activity of Scoparia dulcis Linn. Indian Journal of Pharmaceutical Sciences, 75(5), 610–614.
[24]. Moniruzzaman, M., Atikur Rahman, M., & Ferdous, A., 2015, Evaluation of Sedative and Hypnotic Activity of Ethanolic Extract of Scoparia dulcis Linn. Evidence-based complementary and alternative medicine: eCAM, 2015, 873954. https://doi.org/10.1155/2015/873954.
[25]. Benarfa, A., Gourine, N., Mahfoudi, R., Harrat, M., & Yousfi, M., 2019, Effect of Seasonal and Regional Variations on Phenolic Compounds of Deverra scoparia (Flowers/Seeds) Methanolic Extract and the Evaluation of Its in Vitro Antioxidant Activity. Chemistry & Biodiversity, 16(11), e1900420. https://doi.org/10.1002/cbdv.201900420.
[26]. Coulibaly, A. Y., Kiendrebeogo, M., Kehoe, P. G., Sombie, P. A., Lamien, C. E., Millogo, J. F., & Nacoulma, O. G., 2011, Antioxidant and anti-inflammatory effects of Scoparia dulcis L. Journal of Medicinal Food, 14(12), 1576–1582. https://doi.org/10.1089/jmf.2010.0191.
[27]. Yasothkumar, D., Ramani, P., Jayaraman, S., Ramalingam, K., & Tilakaratne, W. M., 2024, Expression Profile of Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck Pathology, 18(1), 28.
[28]. Sagar, S., Ramani, P., Moses, S., Gheena, S., & Selvaraj, J., 2024, Correlation of salivary cytokine IL-17A and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology, 1-10.
Viewed PDF 216 3 -
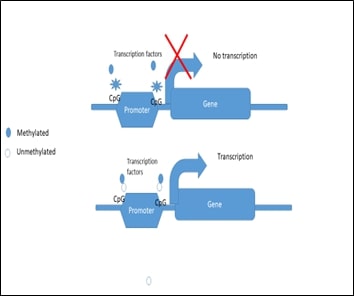 A Comprehensive Review on Impact of Altered Epigenetics on the Development of DiabetesAuthor: Ponnulakshmi RajagopalDOI: 10.21522/TIJPH.2013.SE.25.01.Art014
A Comprehensive Review on Impact of Altered Epigenetics on the Development of DiabetesAuthor: Ponnulakshmi RajagopalDOI: 10.21522/TIJPH.2013.SE.25.01.Art014A Comprehensive Review on Impact of Altered Epigenetics on the Development of Diabetes
Abstract:
In earlier days, researchers were concluding the origin of the disease based on genetic or environmental factors. In the past few years, Epigenetics has been considered as source of certain diseases which could not be ascertained by traditional sources. Recently, more focus has been given to epigenetics, for diseases for which autoimmune disorders, Cardiovascular disease, Cancer, Diabetes, neurodegenerative etc. The original and categorical descriptions of epigenetic alterations, as well as the function of epigenetics in biology and the relationship between epigenetics and the environment, are clarified in the current review. It appears that the significance of epigenetics in human disease is examined by concentrating on a few diseases with complex characteristics. Finally, we have provided an outlook for this field’s future. This review explains the relationship between the epigenetic markers and the environment which influences diabetes.
A Comprehensive Review on Impact of Altered Epigenetics on the Development of Diabetes
References:
[1]. Armstrong, L., 2014, Epigenetics. London: Garland Science.
[2]. Berger, S. L., Kouzarides T., Shiekhattar R., Shilatifard A., 2009, An operational definition of epigenetics. Genes Dev., 1;23 (7), 781–3. doi: 10.1101/gad.1787609.
[3]. Moosavi, A., Ardekani, A. M., 2016, Role of epigenetics in biology and human diseases. Iran. Biomed. J., 20, 246–258.
[4]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P., Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), pp.1007-1013.
[5]. Holliday, R., 2006, Epigenetics: a historical overview. Epigenetics, 1, 76– 80.
[6]. Pinel, C., Prainsack, B., McKevitt, C., 2019, Markers as mediators: A review and synthesis of epigenetics literature. Biosocieties., 10;13(1), 276-303. doi: 10.1057/s41292-017-0068-x.
[7]. Cavalli, G., Heard E., 2019, Advances in epigenetics link genetics to the environment and disease. Nature, 571(7766), 489–499. doi: 10.1038/s41586-019-1411-0.
[8]. Sruthi, M. A., Mani, G., Ramakrishnan, M. and Selvaraj, J., 2023, Dental caries as a source of Helicobacter pylori infection in children: An RT‐PCR study. International Journal of Paediatric Dentistry, 33(1), pp.82-88.
[9]. Bansal, A., Pinney, S. E., 2017, DNA methylation and its role in the pathogenesis of diabetes. Pediatr Diabetes, 18(3), 167–177. doi: 10.1111/pedi.12521.
[10]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P., and Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), pp.704-713.
[11]. Rönn, T., Ling, C., 2015, DNA methylation as a diagnostic and therapeutic target in the battle against Type 2 diabetes. Epigenomics. 7(3):451-60. doi: 10.2217/epi.15.7.
[12]. Szabó, M., Máté, B., Csép, K., Benedek, T., 2018, Epigenetic Modifications Linked to T2D, the Heritability Gap, and Potential Therapeutic Targets. Biochem Genet. 2018 56(6):553-574. doi: 10.1007/s10528-018-9863-8.
[13]. Ling, C., 2020, Epigenetic regulation of insulin action and secretion - role in the pathogenesis of type 2 diabetes. J Intern Med. 288(2):158-167. doi: 10.1111/joim.13049.
[14]. Jin, B., Li, Y., Robertson, K. D., 2011, DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer, 2(6), 607–17. doi: 10.1177/1947601910393957.
[15]. Ling C., 2020, Epigenetic regulation of insulin action and secretion - role in the pathogenesis of type 2 diabetes, J Intern Med, 288(2), 158–167.
[16]. Juvinao-Quintero, D. L., Marioni, R. E., Ochoa-Rosales, C., Russ, T. C., Deary, I. J., Van Meurs, J. B., Voortman, T., Hivert, M. F., Sharp, G. C., Relton, C. L., and Elliott, H. R., 2021, DNA methylation of blood cells is associated with prevalent type 2 diabetes in a meta-analysis of four European cohorts, Clinical epigenetics, 13, 1–14.
[17]. Raciti G. A., Desiderio, A., Longo, M., Leone, A., Zatterale, F., Prevenzano, I., Miele, C., Napoli, R., Beguinot, F., 2021, DNA Methylation and Type 2 Diabetes: Novel Biomarkers for Risk Assessment? Int J Mol Sci., 28;22(21), 11652. doi: 10.3390/ijms222111652.
[18]. Alaskhar A. B., Khalaila, R., Wolf, J., von Bülow V., Harb, H., Alhamdan, F., Hii, C. S., Prescott, S. L., Ferrante, A., Renz, H., Garn, H., Potaczek, D. P., 2018, Histone modifications and their role in epigenetics of atopy and allergic diseases, Allergy Asthma Clin Immunol., 23(14), 39. doi: 10.1186/s13223-018-0259-4.
[19]. Kourtidou, C., Tziomalos, K., 2023, The Role of Histone Modifications in the Pathogenesis of Diabetic Kidney Disease. Int. J. Mol. Sci, 24, 6007, https://doi.org/10.3390/ijms24066007
[20]. Lu, H. C., Dai, W. N., He, L. Y., 2021, Epigenetic Histone Modifications in the Pathogenesis of Diabetic Kidney Disease. Diabetes Metab Syndr Obes, 14, 329-344 https://doi.org/10.2147/DMSO.S288500
[21]. O’Brien, J., Hayder, H., Zayed, Y., Peng, C., 2018, Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne), 9, 402. doi: 10.3389/fendo.2018.00402.
[22]. Wightman, B., Ha, I., Ruvkun, G., 1993, Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell, 75, 855–62. doi: 10.1016/0092-8674(93)90530-4
[23]. Lee, R. C., Feinbaum, R. L., Ambros, V., 1993, The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell, 75, 843–54. doi: 10.1016/0092-8674(93)90529-Y.
[24]. Jo, S., Chen, J., Xu, G., Grayson, T. B., Thielen, L. A., Shalev, A., 2018, miR-204 Controls Glucagon-Like Peptide 1 Receptor Expression and Agonist Function. Diabetes, 67(2), 256–264. doi: 10.2337/db17-0506.
[25]. Bartel, D. P., Metazoan MicroRNAs, 2018, Cell, 173(1), 20–51. doi: 10.1016/j.cell.2018.03.006.
[26]. Denli, A. M., Tops, B. B., Plasterk, R. H., KettingM R. F., Hannon, G. J., 2004, Processing of primary microRNAs by the Microprocessor complex, Nature, 432, 231–5. doi: 10.1038/nature03049.
[27]. Yoda, M., Kawamata, T., Paroo, Z., Ye, X., Iwasaki, S., Liu, Q., et al. 2010, ATP-dependent human RISC assembly pathways, Nat Struct Mol Biol, 17, 17–23. doi: 10.1038/nsmb.1733
[28]. Vargas, E., Podder, V., Carrillo Sepulveda, M. A., Physiology, Glucose Transporter Type 4. 2023, In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
[29]. Ramalingam, K., Yadalam, P. K., Ramani, P., Krishna, M., Hafedh, S., Badnjević, A., Cervino, G., & Minervini, G., 2024, Light gradient boosting-based prediction of quality of life among oral cancer-treated patients. BMC Oral Health, 24(1), 349. https://doi.org/10.1186/s12903-024-04050-x
[30]. Sagar, S., Ramani, P., Moses, S., Gheena, S. and Selvaraj, J., 2024, Correlation of salivary cytokine IL-17A and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology, pp.1-10.
[31]. Neralla, M. M. H., Preethi, A., Selvakumar, S. C., & Sekar, D., 2024, Expression levels of microRNA-7110 in oral squamous cell carcinoma. Minerva Dental and Oral Science, 73(3), 155–160. https://doi.org/10.23736/S2724-6329.23.04801-5
[32]. Erik A. R., 2021, Is GLUT4 translocation the answer to exercise-stimulated muscle glucose uptake? American Journal of Physiology-Endocrinology and Metabolism, 320(2), E240–E243.
[33]. Kaimala, S, Kumar, C. A., Allouh, M. Z., Ansari, S. A., Emerald, B. S., 2022, Epigenetic modifications in pancreas development, diabetes, and therapeutics. Med Res Rev, 42(3), 1343–1371. doi: 10.1002/med.21878.
[34]. Ramsundar, K., Jain, R. K., Balakrishnan, N., & Vikramsimha, B., 2023, Comparative evaluation of bracket bond failure rates of a novel non-primer adhesive with a conventional primer-based orthodontic adhesive - a pilot study. Journal of Dental Research, Dental Clinics, Dental Prospects, 17(1), 35–39. https://doi.org/10.34172/joddd.2023.36953.
[35]. Andersen, M. K., Pedersen, C. E., Moltke, I., Hansen, T., Albrechtsen, A., Grarup, N., 2016, Genetics of Type 2 Diabetes: The Power of Isolated Populations, Curr. Diab. Rep, 16, 65.
[36]. Cole, J. B., Florez, J. C., 2020, Genetics of diabetes mellitus and diabetes complications, Nat. Rev. Nephrol., 16, 377–390.
[37]. Udler, M. S., McCarthy, M. I., Florez, J. C., Mahajan, A., 2019, Genetic Risk Scores for Diabetes Diagnosis and Precision Medicine, Endocrine Rev, 40, 1500–1520.
[38]. Mahajan, A., Taliun, D., Thurner, M., Robertson, N. R., Torres, J. M., Rayner, N. W., Payne, A. J., Steinthorsdottir, V., Scott, R. A., Grarup, N.; et al., 2018, Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps, Nat. Genet, 50, 1505–1513.
[39]. Fathima, J. S., Jayaraman, S., Sekar, R. and Syed, N. H., 2024, The role of MicroRNAs in the diagnosis and treatment of oral premalignant disorders. Odontology, pp.1-10.
[40]. Florez, J. C., Udler, M. S., Hanson, R. L., Genetics of Type 2 Diabetes, 2018, In Diabetes in America, 3rd ed.; Cowie, C.C., Casagrande, S.S., Menke, A., Cissell, M.A., Eberhardt, M.S., Meigs, J.B., Gregg, E.W., Knowler, W.C., Barrett-Connor, E., Becker, D.J., et al., Eds.; National Institute of Diabetes and Digestive and Kidney Diseases (US): Bethesda, MA, USA, pp. 1–25, Chapter 14.
[41]. Florez, J. C., 2008, Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: Where are the insulin resistance genes? Diabetologia, 51, 1100–1110.
[42]. McCarthy, M. I., 2010, Genomics, type 2 diabetes, and obesity. N. Engl. J. Med, 363, 2339–2350.
[43]. Petrie, J. R., Pearson, E. R., Sutherland, C., 2011, Implications of genome wide association studies for the understanding of type 2 diabetes pathophysiology. Biochem. Pharmacol, 81, 471–477.
[44]. Altshuler, D., Hirschhorn, J. N., Klannemark, M., Lindgren, C. M., Vohl, M. C., Nemesh, J., Lane, C.R., Schaffner, S. F., Bolk, S., Brewer, C., et al., 2000, The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat. Genet., 26, 76–80.
[45]. Gloyn, A. L., Weedon, M. N., Owen, K. R., Turner, M. J., Knight, B. A., Hitman, G., Walker, M., Levy, J. C., Sampson, M., Halford, S., et al. 2003, Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes, Diabetes, 52, 568–572.
[46]. Chi, T., Lin, J., Wang, M., Zhao, Y., Liao, Z., Wei, P., 2021, Non-Coding RNA as Biomarkers for Type 2 Diabetes Development and Clinical Management. Front Endocrinol (Lausanne). 12:630032. doi: 10.3389/fendo.2021.630032.
[47]. Fanucchi, S., Domínguez-Andrés, J., Joosten, L. A. B., Netea, M. G., Mhlanga, M. M., 2021, The Intersection of Epigenetics and Metabolism in Trained Immunity. Immunity, 54(1), 32–43. doi: 10.1016/j.immuni.2020.10.011.
[48]. Krishnan, R. P., Pandiar, D., Ramani, P. and Jayaraman, S., 2025, Molecular profiling of oral epithelial dysplasia and oral squamous cell carcinoma using next generation sequencing. Journal of Stomatology, Oral and Maxillofacial Surgery, 126(4), p.102120.
[49]. Čugalj, K. B., Trebušak, K., Kovač, J., Šket, R., Jenko, B. B., Tesovnik, T., Debeljak, M., Battelino, T., Bratina, N., 2022, The Role of Epigenetic Modifications in Late Complications in Type 1 Diabetes. Genes (Basel), 13(4), 705. doi: 10.3390/genes13040705.
[50]. Singh, R., Chandel, S., Dey, D., Ghosh, A., Roy, S., Ravichandiran, V., Ghosh, D., 2020, Epigenetic modification and therapeutic targets of diabetes mellitus. Biosci Rep. 40(9): BSR20202160. doi: 10.1042/BSR20202160.
[51]. Paneni, F., Costantino, S., Battista, R., Castello, L., Capretti, G., Chiandotto, S., Scavone, G., Villano, A., Pitocco, D., Lanza, G., Volpe, M., Lüscher, T. F., Cosentino, F., 2015, Adverse epigenetic signatures by histone methyltransferase Set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ Cardiovasc Genet. 8(1):150-8. doi: 10.1161/CIRCGENETICS.114.000671.
[52]. Yasothkumar, D., Ramani, P., Jayaraman, S., Ramalingam, K., and Tilakaratne, W. M., 2024, Expression Profile of Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck Pathology, 18(1), p.28.
Viewed PDF 231 5 -
 Fabrication and Characterization of Hyaluronic Acid/Tricalcium Phosphate/Quercetin-Doped Silver Membrane for Guided Bone RegenerationAuthor: Gayathri RengasamyDOI: 10.21522/TIJPH.2013.SE.25.01.Art015
Fabrication and Characterization of Hyaluronic Acid/Tricalcium Phosphate/Quercetin-Doped Silver Membrane for Guided Bone RegenerationAuthor: Gayathri RengasamyDOI: 10.21522/TIJPH.2013.SE.25.01.Art015Fabrication and Characterization of Hyaluronic Acid/Tricalcium Phosphate/Quercetin-Doped Silver Membrane for Guided Bone Regeneration
Abstract:
Guided Bone Regeneration (GBR) is a crucial technique in promoting bone growth in areas where bone tissue is deficient. The use of GBR membranes is essential in preventing the resorption of new bone tissue by the body. This study aims to develop an electrospun GBR membrane utilizing Hyaluronic Acid (HA) with Tricalcium Phosphate (TCP) and quercetin-doped silver to explore its potential in promoting bone regeneration. The novel HA/TCP/quercetin-doped silver membrane demonstrated favorable biocompatibility, mechanical properties, and potential to enhance bone growth. This makes it a promising candidate for clinical applications in GBR. The membrane exhibited superior mechanical properties, biocompatibility, and ability to promote bone growth both in vitro and in vivo, indicating its potential as an innovative material for GBR.
Fabrication and Characterization of Hyaluronic Acid/Tricalcium Phosphate/Quercetin-Doped Silver Membrane for Guided Bone Regeneration
References:
[1]. Alqahtani, F., 2023, Periodontal tissue engineering and regeneration: A review of the current literature. J Periodontol, 94(4), 567-579.
[2]. Buser, D., Dula, K., Belser, U. C., Hirt, H. P., Berthold, H., 1995, Localized ridge augmentation using guided bone regeneration. Clin Oral Implants Res, 6(3), 162-168.
[3]. Dahlin, C., Sennerby, L., Lekholm, U., Linde, A., Nyman, S., 1989, Generation of new bone around titanium implants using a membrane technique: An experimental study in rabbits. Int J Oral Maxillofac Implants, 4(1), 19-25.
[4]. Dave, P. H., Vishnupriya, V., Gayathri, R., 2016, Herbal remedies for anxiety and depression—A review. J Adv Pharm Technol Res, 9, 1253.
[5]. Balaji, V., Priya, V. V., Gayathri, R., 2017, Awareness of risk factors for obesity among college students in Tamil Nadu: A questionnaire-based study. J Adv Pharm Technol Res, 10, 1367.
[6]. Gayathri, R., Anuradha, V., 2015, Phytochemical screening and total phenolic content of aqueous and acetone extracts of seed, butter, mace of nutmeg (Myristica fragrans Houtt). Int J Pharm Sci Rev Res.
[7]. Elgali, I., Omar, O., Dahlin, C., Thomsen, P., 2017, Guided bone regeneration: Materials and biological mechanisms revisited. Eur J Oral Sci, 125(5), 315-337.
[8]. Hämmerle, C. H. F., Jung, R. E., Feloutzis, A., 2002, A systematic review of the survival of implants in bone sites augmented with barrier membranes (guided bone regeneration) in partially edentulous patients. J Clin Periodontol, 29(Suppl. 3), 226-231.
[9]. Wang, H. L., Boyapati, L., 2006, "PASS" principles for predictable bone regeneration. Implant Dent, 15(1), 8-17.
[10]. Jerusha, S. P., Gayathri, R., Vishnupriya, V., 2016, Preliminary phytochemical analysis and cytotoxicity potential of Bacopa monnieri on oral cancer cell lines. Int J Pharm Sci Rev Res, 39, 4-8.
[11]. Satyamoorthy, K., Gayathri, R., Bhat, K., Saadi, A., Bhat, S., 2011, Allele, genotype, and composite genotype effects of IL-1A +4845 and IL-1B +3954 polymorphisms for chronic periodontitis in an Indian population. Indian J Dent Res, 22(4), 612.
[12]. Wikesjö, U. M., Polimeni, G., Qahash, M., Susin, C., Shanaman, R. H., Rohrer, M. D., Wozney, J. M., 2009, Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: Histologic observations. J Clin Periodontol, 36(9), 759-766.
[13]. Wang, H. L., Carroll, M. J., 2001, Guided bone regeneration using bone grafts and collagen membranes. Quintessence Int, 32(7), 504-515.
[14]. Nevins, M., Mellonig, J. T., 1992, Enhancement of the damaged edentulous ridge to receive dental implants: A combination of allograft and the Gore-Tex membrane. Int J Periodontics Restorative Dent, 12(2), 96-111.
[15]. Urban, I. A., Monje, A., Wang, H. L., 2019, Horizontal ridge augmentation with guided bone regeneration. Periodontol 2000, 80(1), 152-172.
[16]. Sculean, A., Gruber, R., Bosshardt, D. D., 2014, Soft tissue wound healing around teeth and dental implants. J Clin Periodontol, 41(Suppl. 15).
[17]. Kumar, J. K., Surendranath, P., Eswaramoorthy, R., 2023, Regeneration of immature incisor using platelet-rich fibrin: Report of a novel clinical application. BMC Oral Health, 23, 69.
[18]. Kishen, A., Cecil, A., Chitra, S., 2023, Fabrication of hydroxyapatite-reinforced polymeric hydrogel membrane for regeneration. Saudi Dent J, 35, 678-683.
[19]. Ramamurthy, J., Bajpai, D., 2024, Role of alginate-based scaffolds for periodontal regeneration of intrabony defects: A systematic review. World J Dent, 15, 181-187.
[20]. Tirupathi, S., Afnan, L., 2024, Dental pulp-derived stem cells for facial nerve regeneration and functional repair: A systematic review of animal studies. Curr Oral Health Rep, 11, 198-214.
[21]. Keziah, V. S., Gayathri, R., Priya, V. V., 2018, Biodegradable plastic production from corn starch. Drug Invention Today, 10(7), 1315-1317.
[22]. Hirshasri, A.G., Renu, K., Priya, V.V., Gayathri, R. and Kavitha, S., The Effect of Aspalathin on SMAD2, SMAD3, TGF-β-A Major Contributor of Inflammation–An In-silico Approach.
[23]. Priya, V., Keerthivasan, S. and Kaviyarasi, R., Determining the Dual Effect of Mirabegron on Anticancer Mechanism and Brown Adipose Tissue Activation-An in-silico Approach.
[24]. Juvairiya Fathima, A., Renu, K., Priya, V.V., Gayathri, R. and Kavitha, S., Determining the Role of Caffeic Acid on Lipogenic Regulators: An In-Silico Approach.
[25]. Manoharan, S. A. D., Vishnupriya, V. A. D., Gayathri, R., 2015, Phytochemical analysis and in vitro antioxidant activity of jojoba oil. Int J Pharm Sci Rev Res, 8(6), 512-516.
[26]. Miron, R. J., Bosshardt, D. D., 2018, OsteoMacs: Key players around bone biomaterials. Biomaterials, 195, 176-184.
[27]. Retzepi, M., Donos, N., 2010, Guided bone regeneration: Biological principle and therapeutic applications. Clin Oral Implants Res, 21(6), 567-576.
[28]. Echhpal, U., Shah, K.K., Maiti, S., Raju, L., Eswaramoorthy, R. and Nallaswamy, D., 2024. An In Vitro Study to Evaluate the Cell Viability, Surface Characteristics, and Osteoconductive Potential of Ovine Bone Graft Modified with L-Glutamine Compared to Commercial Bone Graft. International Journal of Prosthodontics and Restorative Dentistry, 14(3), pp.185-193.
[29]. Shankar, P., Arumugam, P. and Kannan, S., 2024. Development, Characterisation and Biocompatibility Analysis of a Collagen-Gelatin-Hydroxyapatite Scaffold for Guided Bone Regeneration. Odovtos-International Journal of Dental Sciences, pp.235-248.
[30]. Franco, R., Cervino, G., Vazzana, G., Della Rocca, F., Ferrari, G., Cicciù, M. and Minervini, G., 2024. Use of Concentrated Growth Factor (CGF) in Prosthetic-Guided Reconstruction on Two-Wall Bone Defect after Cystectomy: An Alternative to Traditional Regeneration. European Journal of Dentistry, 18(01), pp.392-396.
[31]. Awate, S., Bhate, K., Contractor, M.M., Londhe, U., Samuel, S. and Tirupathi, S., 2024. Comparison of Activated PRF and Standard PRF in Terms of Evaluation of Quality Bone Regeneration in Extraction Sockets of Impacted Third Molar: A Split-Mouth Double-Blind Control Trial. Journal of Maxillofacial and Oral Surgery, pp.1-9.
Viewed PDF 206 6 -
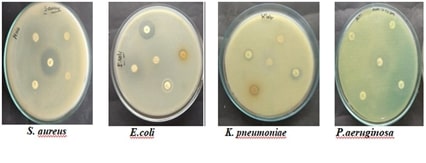 Evaluating the Antimicrobial Efficacy of Solvent Extracts from Gymnema Sylvestre Against Wound PathogensAuthor: Sengottuvel RDOI: 10.21522/TIJPH.2013.SE.25.01.Art016
Evaluating the Antimicrobial Efficacy of Solvent Extracts from Gymnema Sylvestre Against Wound PathogensAuthor: Sengottuvel RDOI: 10.21522/TIJPH.2013.SE.25.01.Art016Evaluating the Antimicrobial Efficacy of Solvent Extracts from Gymnema Sylvestre Against Wound Pathogens
Abstract:
The antibacterial activity of Gymnema sylvestre solvent extracts, including acetone, aqueous, chloroform, ethanol, and hydroxyethanol, is assessed in this study against common wound infections. G. sylvestre is a well-known traditional medicinal herb with a wide range of therapeutic uses that has drawn attention due to its antibacterial actions. The release of bioactive chemicals was optimized by the use of a systematic extraction process with several solvents. Using the agar well diffusion method, the antibacterial activity of the extract was evaluated against a variety of wound pathogens, such as Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus. The findings showed that the antibacterial activity of the various solvent extracts varied significantly. The extracts of ethanol, acetone, chloroform, and hydroxyethanol showed the strongest inhibitory effects, dramatically slowing the growth of the pathogens under examination. Moderate action was demonstrated by aqueous extracts against the acquired infections. These results indicate that the bioavailability of antimicrobial compounds in G. sylvestre is significantly influenced by the extraction solvent. The study emphasizes the potential of G. sylvestre as a natural antibacterial agent, especially about wound healing. The precise bioactive components causing this activity must be determined, and their mechanisms of action must be investigated, through more research. Overall, this research adds to the increasing evidence that supports the use of herbal remedies for managing wound-related infections, providing valuable insights for future therapeutic applications.
Evaluating the Antimicrobial Efficacy of Solvent Extracts from Gymnema Sylvestre Against Wound Pathogens
References:
[1]. Salam, M. A., Al-Amin, M. Y., Salam, M. T., Pawar, J. S., Akhter, N., Rabaan, A. A., Alqumber, M. A., 2023, Antimicrobial resistance: a growing serious threat for global public health. In Healthcare 11(13), 1946.
[2]. Chiș, A. A., Rus, L. L., Morgovan, C., Arseniu, A. M., Frum, A., Vonica-Țincu, A. L., Dobrea, C. M., 2022, Microbial resistance to antibiotics and effective antibiotherapy. Biomedicines, 10(5), 1121.
[3]. Liu, G. Y., Yu, D., Fan, M. M., Zhang, X., Jin, Z. Y., Tang, C., & Liu, X. F., 2024, Antimicrobial resistance crisis: could artificial intelligence be the solution? Military Medical Research, 11(1), 7.
[4]. Patel, K., Patel, D. K., 2017, Medicinal importance, pharmacological activities, and analytical aspects of hispidulin: A concise report. Journal of traditional and complementary medicine, 7(3), 360-366.
[5]. Fatima, N., Baqri, S. S. R., Alsulimani, A., Fagoonee, S., Slama, P., Kesari, K. K., Haque, S., 2021, Phytochemicals from Indian ethnomedicines: Promising prospects for the management of oxidative stress and cancer. Antioxidants, 10(10), 1606.
[6]. Chaughule, R. S., Barve, R. S., 2024, Role of herbal medicines in the treatment of infectious diseases. Vegetos, 37(1), 41-51.
[7]. Courric, E., Brinvilier, D., Couderc, P., Ponce-Mora, A., Méril-Mamert, V., Sylvestre, M., Cebrian-Torrejon, G., 2023, Medicinal plants and plant-based remedies in Grande-Terre: an ethnopharmacological approach. Plants, 12(3), 654.
[8]. Vaou, N., Stavropoulou, E., Voidarou, C., Tsigalou, C., & Bezirtzoglou, E., 2021, Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms, 9(10), 2041.
[9]. Ashraf, M. V., Pant, S., Khan, M. H., Shah, A. A., Siddiqui, S., Jeridi, M., Ahmad, S., 2023, Phytochemicals as antimicrobials: prospecting Himalayan medicinal plants as source of alternate medicine to combat antimicrobial resistance. Pharmaceuticals, 16(6), 881.
[10]. Entooru, K., Krishnaprasad, M. S., Thimmaiah, S. B., Nanjaiah, S. K., Kolgi, R. R., Bopaiah, R. U., Thimmappa, N., 2021, Evaluation of antimicrobial activity of leaf extract of Gymnema sylvestre. IJCS, 9(2), 1123-1125.
[11]. Ramadass, N., Subramanian, N., Ponnulakshmi, R., Selvaraj, J., 2019, Phytochemical Screening and Antibacterial Activity of Leaf Extracts of Gymnema sylvestre against Pathogenic Bacteria. Int. J. Sci. Res. in Biological Sciences Vol, 6, 1.
[12]. Kavipriya, R., Ramasubburayan, R., 2024, Phytofabrication of biocompatible zinc oxide nanoparticle using Gymnema sylvestre and its potent in vitro antibacterial, antibiofilm, and cytotoxicity against human breast cancer cells (MDA-MB-231). Bioprocess and Biosystems Engineering, 1-15.
[13]. Arunachalam, K. D., Arun, L. B., Annamalai, S. K., Arunachalam, A. M., 2015, Potential anticancer properties of bioactive compounds of Gymnema sylvestre and its biofunctionalized silver nanoparticles. International Journal of Nanomedicine, 31-41.
[14]. Subramanian, S., Dowlath, M. J. H., Karuppannan, S. K., Saravanan, M., Arunachalam, K. D., 2020. Effect of solvent on the phytochemical extraction and GC-MS analysis of Gymnema sylvestre. Pharmacognosy Journal, 12(4).
[15]. Kabeerdass, N., Thangasamy, S., Murugesan, K., Arumugam, N., Almansour, A. I., Suresh Kumar, R., Mathanmohun, M., 2022, Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach. Green Processing and Synthesis, 11(1), 875-885.
[16]. Kabeerdass, N., Kandasamy, S., Albasher, G., Alamri, O., Alsultan, N., Thangaswamy, S., Mathanmohun, M., 2022, Limonia acidissima leaf mediated gold nanoparticles synthesis and their antimicrobial and wound healing properties. Materials Letters, 314, 131893.
[17]. Muteeb, G., Rehman, M. T., Shahwan, M., & Aatif, M., 2023, Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals, 16(11), 1615.
[18]. Chaachouay, N., Zidane, L., 2024, Plant-derived natural products: a source for drug discovery and development. Drugs and Drug Candidates, 3(1), 184-207.
[19]. Arora, D. S., Sood, H., 2017, In vitro antimicrobial potential of extracts and phytoconstituents from Gymnema sylvestre R. Br. leaves and their biosafety evaluation. AMB express, 7, 1-13.
[20]. Xu, Z., Li, M., Li, Y., Cao, H., Miao, L., Xu, Z., Yan, A., 2019, Native CRISPR-Cas-mediated genome editing enables dissecting and sensitizing clinical multidrug-resistant P. aeruginosa. Cell Reports, 29(6), 1707-1717.
[21]. Hafiz, T. A., Alanazi, S., Alghamdi, S. S., Mubaraki, M. A., Aljabr, W., Madkhali, N., Alotaibi, F., 2023, Klebsiella pneumoniae bacteraemia epidemiology: resistance profiles and clinical outcome of King Fahad Medical City isolates, Riyadh, Saudi Arabia. BMC Infectious Diseases, 23(1), 579.
[22]. Linz, M. S., Mattappallil, A., Finkel, D., Parker, D. 2023, Clinical impact of Staphylococcus aureus skin and soft tissue infections. Antibiotics, 12(3), 557.
[23]. Zhou, Y., Zhou, Z., Zheng, L., Gong, Z., Li, Y., Jin, Y., Chi, M., 2023, Urinary tract infections caused by uropathogenic Escherichia coli: mechanisms of infection and treatment options. International journal of molecular sciences, 24(13), 10537.
[24]. Gunasekaran, V., Srinivasan, S., Rani, S., 2019, Potential antioxidant and antimicrobial activity of Gymnema sylvestre related to diabetes. J. Med. Plants, 7(2), 05-11.
[25]. Solanki, P., Arora, A., 2024, Isolation and characterization of peptides from Gymnema sylvestre and their antimicrobial assay against bacterial isolates of diabetic foot ulcer. Journal of Pharmacognosy and Phytochemistry, 13(4), 154-160.
[26]. Ganesan, M., Muthaiah, C., Wadaan, M. A., Kumar, M., Yanto, D. H. Y., Kumar, S., Suganthi, S., 2024, Synthesis and characterization of fluorinated graphene oxide nanosheets derived from Lissachatina fulica snail mucus and their biomedical applications. Luminescence, 39(9), e4875.
[27]. Saritha, P., Arunprakash, S., Srinivasan, P., Selvankumar, T., Aldawood, S., Kim, W., Song, K. S., 2024, Synthesis of Luminescent Copper Nanoparticles Using Couroupita guianensis Flower Extract: Evaluation of Antibacterial and Anticancer Activities. Luminescence, 39(10), e4913.
[28]. Abareethan, M., Sathiyapriya, R., Pavithra, M. E., Parvathy, S., Thirumalaisamy, R., Selvankumar, T., Almoallim, H. S., 2024, Biogenic Silver Nanoparticles from Solanum trilobatum Leaf Extract and Assessing their Antioxidant and Antimicrobial Potential. Chemical Physics Impact, 100771.
Viewed PDF 261 19 -
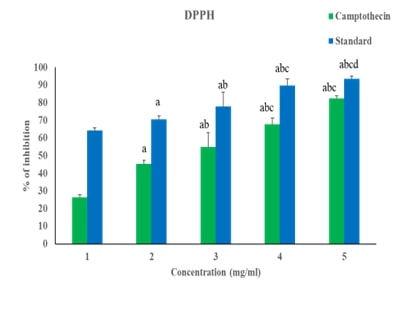 Camptothecin Anti-cancer Activity Against Breast Cancer Cells (MDA MB-231) Targeting the Gene Expression of Wnt/Beta-catenin Pathway - An In silico and In vitro ApproachAuthor: Selvaraj JayaramanDOI: 10.21522/TIJPH.2013.SE.25.01.Art017
Camptothecin Anti-cancer Activity Against Breast Cancer Cells (MDA MB-231) Targeting the Gene Expression of Wnt/Beta-catenin Pathway - An In silico and In vitro ApproachAuthor: Selvaraj JayaramanDOI: 10.21522/TIJPH.2013.SE.25.01.Art017Camptothecin Anti-cancer Activity Against Breast Cancer Cells (MDA MB-231) Targeting the Gene Expression of Wnt/Beta-catenin Pathway - An In silico and In vitro Approach
Abstract:
Camptothecin, a potent anti-cancer agent, exhibits significant activity against MDA-MB-231 breast cancer cells by targeting the gene expression of the Wnt/β-catenin pathway. This pathway is crucial in cancer progression and cell proliferation. Camptothecin's effect on this pathway is elucidated through various assays and docking techniques. The DPPH assay demonstrates camptothecin's antioxidant potential, indicating its ability to neutralize free radicals. Additionally, nitric oxide assays reveal a significant enhancement in antioxidant properties, further supporting its therapeutic potential. Gene expression analysis provides insights into the molecular mechanisms underlying camptothecin's anti-cancer effects. The expression levels of key components of the Wnt/β-catenin pathway, including Wnt, β-catenin, APC, GSK3β, LP5, and Axin, are significantly altered in MDA-MB-231 cells upon camptothecin treatment. These changes suggest a disruption in the signaling pathway, which is vital for cancer cell survival and proliferation. The MTT assay results highlight camptothecin's capacity to inhibit cell growth in a time-dependent manner, underscoring its efficacy in reducing cancer cell viability over prolonged exposure. Moreover, docking studies indicate a high binding affinity between camptothecin and the Wnt/β-catenin pathway components, reinforcing the compound's role in modulating this critical signaling axis. Overall, camptothecin's multi-faceted approach, encompassing antioxidant activity and targeted gene expression modulation, presents a compelling case for its use in breast cancer therapy. The comprehensive analysis of its effects on the Wnt/β-catenin pathway offers valuable insights into its mechanism of action and potential as a therapeutic agent against aggressive breast cancer types like MDA-MB-231 cells.
Camptothecin Anti-cancer Activity Against Breast Cancer Cells (MDA MB-231) Targeting the Gene Expression of Wnt/Beta-catenin Pathway - An In silico and In vitro Approach
References:
[1]. Venditto, V. J., & Simanek, E. E., 2010, Cancer therapies utilizing the camptothecins: a review of the in vivo literature. Molecular Pharmaceutics, 7(2), 307–349. https://doi.org/10.1021/mp900243b
[2]. Narasimhan, A., Sampath, S., Jayaraman, S., & Karundevi, B., 2013, Estradiol favors glucose oxidation in gastrocnemius muscle through modulation of insulin signaling molecules in adult female rats. Endocrine Research, 38(4), 251-262.
[3]. Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Veeraraghavan, V. P., 2022, Effect of Carica papaya on IRS-1/Akt signaling mechanisms in high-fat-diet-streptozotocin-induced type 2 diabetic experimental rats: A mechanistic approach. Nutrients, 14(19), 4181.
[4]. Perumal, S., Langeshwaran, K., Selvaraj, J., Ponnulakshmi, R., Shyamaladevi, B., & Balasubramanian, M. P., 2018, Effect of diosmin on apoptotic signaling molecules in N-nitrosodiethylamine-induced hepatocellular carcinoma in experimental rats. Molecular and Cellular Biochemistry, 449, 27-37.
[5]. Devarajan, N., Jayaraman, S., Mahendra, J., Venkatratnam, P., Rajagopal, P., Palaniappan, H., & Ganesan, S. K., 2021, Berberine—A potent chemosensitizer and chemoprotector to conventional cancer therapies. Phytotherapy Research, 35(6), 3059-3077.
[6]. Jayaraman, S., Natararaj, S., & Veeraraghavan, V. P., 2024, Hesperidin inhibits oral cancer cell growth via apoptosis and inflammatory signaling-mediated mechanisms: Evidence from in vitro and in silico analyses. Cureus, 16(2).
[7]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P., & Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), 704-713.
[8]. Jayaraman, S., Veeraraghavan, V. P., Natarajan, S. R., & Jasmine, S., 2024, Exploring the therapeutic potential of curcumin in oral squamous cell carcinoma (HSC-3 cells): Molecular insights into hypoxia-mediated angiogenesis. Pathology-Research and Practice, 254, 155130.
[9]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P., & Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), 1007-1013. https://doi.org/10.1016/j.sdentj.2023.08.003
[10]. Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Veeraraghavan, V. P., 2022, Carica papaya reduces muscle insulin resistance via IR/GLUT4 mediated signaling mechanisms in high fat diet and streptozotocin-induced type-2 diabetic rats. Antioxidants, 11(10), 2081.
[11]. Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Prasad, M., 2023, Carica papaya reduces high fat diet and streptozotocin-induced development of inflammation in adipocyte via IL-1β/IL-6/TNF-α mediated signaling mechanisms in type-2 diabetic rats. Current Issues in Molecular Biology, 45(2), 852.
[12]. Baliyan, S., Mukherjee, R., Priyadarshini, A., Vibhuti, A., Gupta, A., Pandey, R. P., & Chang, C. M., 2022, Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules (Basel, Switzerland), 27(4), 1326. https://doi.org/10.3390/molecules27041326
[13]. Chen, K., Pittman, R. N., & Popel, A. S., 2008, Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxidants & Redox Signaling, 10(7), 1185–1198. https://doi.org/10.1089/ars.2007.1959
[14]. Krishnamoorthy, K., Natarajan, S. R., Veeraraghavan, V. P., & Jayaraman, S., 2024, Blueberry extract and its bioactive compounds mitigate oxidative stress and suppress human lung cancer cell (A549) growth by modulating the expression of p53/EGFR/STAT3/IL6-mediated signaling molecules. Cell Biochemistry and Function, 42(4), e4027. https://doi.org/10.1002/cbf.4027
[15]. Indu, S., Vijayalakshmi, P., Selvaraj, J., & Rajalakshmi, M., 2021, Novel triterpenoids from Cassia fistula stem bark depreciates STZ-induced detrimental changes in IRS-1/Akt-mediated insulin signaling mechanisms in type-1 diabetic rats. Molecules, 26(22), 6812.
[16]. Deenadayalan, A., Subramanian, V., Paramasivan, V., Veeraraghavan, V. P., Rengasamy, G., Coiambatore Sadagopan, J., & Jayaraman, S., 2021, Stevioside attenuates insulin resistance in skeletal muscle by facilitating IR/IRS-1/Akt/GLUT 4 signaling pathways: An in vivo and in silico approach. Molecules, 26(24), 7689.
[17]. Chang, L. C., Chen, T. C., Chen, S. J., Chen, C. L., Lee, C. C., Wu, S. H., & Lin, J. J., 2016, Identification of a new class of WNT1 inhibitor: Cancer cells migration, G-quadruplex stabilization and target validation. Oncotarget, 7(42), 67986.
[18]. Khademian, N., Mirzaei, A., Hosseini, A., Zare, L., Nazem, S., Babaheidarian, P., & Tavakoli-Yaraki, M., 2022, Expression pattern and clinical significance of β-catenin gene and protein in patients with primary malignant and benign bone tumors. Scientific Reports, 12(1), 9488.
[19]. Domoto, T., Uehara, M., Bolidong, D., & Minamoto, T., 2020, Glycogen synthase kinase 3β in cancer biology and treatment. Cells, 9(6), 1388.
[20]. Prasad, M., Gatasheh, M. K., Alshuniaber, M. A., Krishnamoorthy, R., Rajagopal, P., Krishnamoorthy, K., et al. 2022, Impact of Glyphosate on the Development of Insulin Resistance in Experimental Diabetic Rats: Role of NFκB Signalling Pathways. Antioxidants (Basel), 11(12), 2436. http://dx.doi.org/10.3390/antiox11122436
[21]. Vishaka, S., Sridevi, G., Selvaraj, J., 2022, An in vitro analysis on the antioxidant and anti-diabetic properties of Kaempferia galanga rhizome using different solvent systems. J Adv Pharm Technol Res, 13(Suppl 2), S505–9. http://dx.doi.org/10.4103/japtr.japtr_189_22
[22]. Chandran, D., Jayaraman, S., Sankaran, K., Veeraraghavan, V. P., Gayathri, R., 2023, Antioxidant Vitamins Attenuate Glyphosate-Induced Development of Type-2 Diabetes Through the Activation of Glycogen Synthase Kinase-3 β and Forkhead Box Protein O-1 in the Liver of Adult Male Rats. Cureus, 15(12), e51088. doi: 10.7759/cureus.51088.
[23]. El-Sayed, A. S. A., Hassan, W. H. B., Sweilam, S. H., Alqarni, M. H. S., El Sayed, Z. I., Abdel-Aal, M. M., Abdelsalam, E., & Abdelaziz, S., 2022, Production, Bioprocessing and Anti-Proliferative Activity of Camptothecin from Penicillium chrysogenum, "An Endozoic of Marine Sponge, Cliona sp.", as a Metabolically Stable Camptothecin Producing Isolate. Molecules (Basel, Switzerland), 27(9), 3033. https://doi.org/10.3390/molecules27093033.
[24]. Yadalam, P. K., Arumuganainar, D., Ronsivalle, V., Di Blasio, M., Badnjevic, A., Marrapodi, M. M., Cervino, G., & Minervini, G., 2024, Prediction of interactomic hub genes in PBMC cells in type 2 diabetes mellitus, dyslipidemia, and periodontitis. BMC oral health, 24(1), 385. https://doi.org/10.1186/s12903-024-04041-y.
[25]. Al-Shorman, H. M., Abu-Naba'a, L. A., Sghaireen, M. G., & Alam, M. K., 2024, The Effect of Various Preparation and Cementation Techniques of Dental Veneers on Periodontal Status: A Systematic Review and Meta-Analysis. European Journal of Dentistry, 18(2), 458–467. https://doi.org/10.1055/s-0043-1776120.
[26]. Mathew, M. G., Jeevanandan, G., Vishwanathaiah, S., Hamzi, K. A., Depsh, M. A. N., & Maganur, P. C., 2022, Parental and Child Outlook on the Impact of ECC on Oral Health-related Quality of Life: A Prospective Interventional Study. The Journal of Contemporary Dental Practice, 23(9), 877–882. https://doi.org/10.5005/jp-journals-10024-3397
[27]. Jamshidi-Adegani, F., Ghaemi, S., Al-Hashmi, S., Vakilian, S., Al-Kindi, J., Rehman, N. U., Alam, K., Al-Riyami, K., Csuk, R., Arefian, E., & Al-Harrasi, A., 2022, Comparative study of the cytotoxicity, apoptotic, and epigenetic effects of Boswellic acid derivatives on breast cancer. Scientific Reports, 12(1), 19979. https://doi.org/10.1038/s41598-022-24229-y
[28]. Hemmati Bushehri, R., Navabi, P., Saeedifar, A. M., Keshavarzian, N., Hosseini Rouzbahani, N., Mosayebi, G., Ghazavi, A., Ghorban, K., & Ganji, A., 2023, Integration of phytotherapy and chemotherapy: Recent advances in anticancer molecular pathways. Iranian Journal of Basic Medical Sciences, 26(9), 987–1000. https://doi.org/10.22038/IJBMS.2023.69979.15222.
[29]. Lopes, C. M., Dourado, A., & Oliveira, R., 2017, Phytotherapy and Nutritional Supplements on Breast Cancer. BioMed Research International, 2017, 7207983. https://doi.org/10.1155/2017/7207983.
[30]. Bailly, C., & Vergoten, G., 2023, Interaction of Camptothecin Anticancer Drugs with Ribosomal Proteins L15 and L11: A Molecular Docking Study. Molecules (Basel, Switzerland), 28(4), 1828. https://doi.org/10.3390/molecules28041828.
[31]. Wintola, O. A., & Afolayan, A. J., 2011, Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox Mill. Pharmacognosy Magazine, 7(28), 325–333. https://doi.org/10.4103/0973-1296.90414.
[32]. Simu, S., Marcovici, I., Dobrescu, A., Malita, D., Dehelean, C. A., Coricovac, D., Olaru, F., Draghici, G. A., & Navolan, D., 2021, Insights into the Behavior of Triple-Negative MDA-MB-231 Breast Carcinoma Cells Following the Treatment with 17β-Ethinylestradiol and Levonorgestrel. Molecules (Basel, Switzerland), 26(9), 2776. https://doi.org/10.3390/molecules26092776.
[33]. Márquez-Garbán, D. C., Yanes, C. D., Llarena, G., Elashoff, D., Hamilton, N., Hardy, M., Wadehra, M., McCloskey, S. A., & Pietras, R. J., 2024, Manuka Honey Inhibits Human Breast Cancer Progression in Preclinical Models. Nutrients, 16(14), 2369. https://doi.org/10.3390/nu16142369
Viewed PDF 230 2 -
 A Study to Assess the Effectiveness of Structured Teaching Program on Knowledge on Hand Washing among School Children at Selected SchoolAuthor: Ajith MDOI: 10.21522/TIJPH.2013.SE.25.01.Art018
A Study to Assess the Effectiveness of Structured Teaching Program on Knowledge on Hand Washing among School Children at Selected SchoolAuthor: Ajith MDOI: 10.21522/TIJPH.2013.SE.25.01.Art018A Study to Assess the Effectiveness of Structured Teaching Program on Knowledge on Hand Washing among School Children at Selected School
Abstract:
The study aimed to evaluate the effectiveness of structured teaching programmes on knowledge regarding hand washing techniques and their importance among school-going children at selected schools Namakkal district. electronic hand hygiene systems have emerged to not only record compliance but also to promote it among HCWs. Modifications of the hand hygiene procedure have been proposed targeting both time and technique of hand rub application. Reducing rub time from 30 to 15 s and simplifying the technique to consist of three rather than six steps encourages results in terms of microbiological efficacy and compliance. Methods. A community-based comparative cross-sectional study was conducted in Namakkal, District. The study population consisted of schoolgoers who studying in primary school. The instrument used in this study was a self-structured knowledge questionnaire. The collected data was organized tabulated, summarized and analyzed by using descriptive and inferential statistics. The data were analyzed by using the Chi-square test and were calculated to analyze the differences in pre-test and post-test scores on the level of knowledge before and after the structured teaching program. The statistical paired ‘t' test indicates that the mean effectiveness was found to be significant at p>0.05 revealing that the administration of a structured teaching programme was effective in improving knowledge regarding hands-washing techniques among school going children. Conclusion. There was significant improvement in post-test levels of knowledge when compared to pretest levels. The structured teaching programmes are effective in improving the knowledge about hand washing.
A Study to Assess the Effectiveness of Structured Teaching Program on Knowledge on Hand Washing among School Children at Selected School
References:
[1]. Dagne, H., Bogale, L., Borcha, M., Tesfaye, A., & Dagnew, B. 2019, Hand washing practice at critical times and its associated factors among mothers of under five children in Debark town, northwest Ethiopia, 2018. Italian journal of pediatrics, 45, 1-7.
[2]. Almoslem, M. M., Alshehri, T. A., Althumairi, A. A., Aljassim, M. T., Hassan, M. E., & Berekaa, M. M. 2021, Handwashing knowledge, attitudes, and practices among students in Eastern Province schools, Saudi Arabia. Journal of Environmental and Public Health, 2021(1), 6638443.
[3]. Nagar, K., Darji, J., Parmar, S., Patel, N., & Patel, N. 2021, A Cross Sectional Study to Assess the Knowledge, Attitude and Practice on Personal Hygiene among School Children in Rural Primary School of Kheda District, Gujarat. Indian Journal of Forensic Medicine & Toxicology, 15(3), 290-295.
[4]. Ogwezzy-Ndisika, A. O., & Solomon, T. 2019, Knowledge, attitude and practice of hand washing among mothers of children 0-59 months of age in Lagos Nigeria. Univ J Public Health, 7(2), 52-58.
[5]. Pratinidhi, S. A., Haribhakta, S. V., Ambike, D. A., Bhole, O., & Kankariya, B. 2020, Study of knowledge and practices related to handwashing in school going children of a rural community. International Journal of Contemporary Pediatrics, 7(1), 24.
[6]. Boyce, J. M. 2008, Hand hygiene compliance monitoring: current perspectives from the USA. Journal of Hospital infection, 70, 2-7.
[7]. Bhat, S. R., Achars Textbook of Pediatrics (4Th Edn). Universities Press.
[8]. Beevi, T. A., 2009, Textbook of paediatric nursing. Elsevier.
[9]. Cowie, H., 2019, From birth to sixteen: Children's health, social, emotional and linguistic development. Routledge.
[10]. Taddese, A. A., Dagnew, B., Dagne, H., & Andualem, Z. 2020, Mother’s handwashing practices and health outcomes of under-five children in northwest Ethiopia. Pediatric health, medicine and therapeutics, 101-108.
[11]. Eshetu, D., Kifle, T., & Hirigo, A. T. 2020, Knowledge, attitudes, and practices of hand washing among aderash primary schoolchildren in Yirgalem Town, Southern Ethiopia. Journal of Multidisciplinary Healthcare, 759-768.
[12]. Besha, B., Guche, H., Chare, D., Amare, A., Kassahun, A., Kebede, E., & Yesuf, A. 2016, Assessment of hand washing practice and it’s associated factors among first cycle primary school children in Arba Minch town, Ethiopia, 2015. Epidemiology (Sunnyvale), 6(247), 2161.
[13]. Buda, A. S., Mekengo, D. E., Lodebo, T. M., Sadore, A. A., & Mekonnen, B. 2018, Knowledge, attitude and practice on hand washing and associated factors among public primary schools children in Hosanna town, Southern Ethiopia. Journal of Public Health and Epidemiology, 10(6), 205-214.
[14]. Solomon, E. T., Gari, S. R., Kloos, H., & Alemu, B. M. 2021, Handwashing effect on diarrheal incidence in children under 5 years old in rural eastern Ethiopia: a cluster randomized controlled trial. Tropical medicine and health, 49, 1-11.
[15]. Regassa, W., & Lemma, S. 2016, Assessment of diarrheal disease prevalence and associated risk factors in children of 6–59 months old at Adama District rural Kebeles, eastern Ethiopia, January/2015. Ethiopian journal of Health Sciences, 26(6), 581.
[16]. Adane, M., Mengistie, B., Mulat, W., Medhin, G., & Kloos, H. 2018, The most important recommended times of hand washing with soap and water in preventing the occurrence of acute diarrhea among children under five years of age in slums of Addis Ababa, Ethiopia. Journal of Community Health, 43, 400-405.
[17]. Contzen, N., Meili, I. H., & Mosler, H. J. 2015, Changing handwashing behaviour in southern Ethiopia: a longitudinal study on infrastructural and commitment interventions. Social science & medicine, 124, 103-114.
[18]. Shehmolo, M., Gari, T., Jember Tesfaye, D., Boti, N., & Oumer, B. 2021, Magnitude and factors associated with hygiene practice among primary school children in mareko district, southern Ethiopia: a cross-sectional study. Journal of multidisciplinary healthcare, 311-320.
[19]. Datta, S. S., Singh, Z., Boratne, A. V., Senthilvel, V., Bazroy, J., & Dimri, D. 2011, Knowledge and practice of handwashing among mothers of under five children in rural coastal South India.
[20]. Contzen, N., & Mosler, H. J. 2015, Identifying the psychological determinants of handwashing: results from two cross-sectional questionnaire studies in Haiti and Ethiopia. American journal of infection control, 43(8), 826-832.
[21]. Hashi, A., Kumie, A., & Gasana, J. 2017, Hand washing with soap and WASH educational intervention reduces under-five childhood diarrhoea incidence in Jigjiga District, Eastern Ethiopia: a community-based cluster randomized controlled trial. Preventive medicine reports, 6, 361-368.
[22]. Dagnew, A. B., Tewabe, T., Miskir, Y., Eshetu, T., Kefelegn, W., Zerihun, K., & Teka, T. 2019, Prevalence of diarrhea and associated factors among under-five children in Bahir Dar city, Northwest Ethiopia, 2016: a cross-sectional study. BMC infectious diseases, 19, 1-7.
[23]. Steiner-Asiedu, M., Van-Ess, S. E., Papoe, M., Setorglo, J., Asiedu, D. K., & Anderson, A. K. 2011, Hand washing practices among school children in Ghana.
[24]. Mbakaya, B. C., Lee, P. H., & Lee, R. L. 2017, Hand hygiene intervention strategies to reduce diarrhoea and respiratory infections among schoolchildren in developing countries: a systematic review. International journal of environmental research and public health, 14(4), 371.
[25]. Workie, H. M., Sharifabdilahi, A. S., & Addis, E. M. 2018, Mothers’ knowledge, attitude and practice towards the prevention and home-based management of diarrheal disease among under-five children in Diredawa, Eastern Ethiopia, 2016: a cross-sectional study. BMC pediatrics, 18, 1-9.
[26]. Melese, B., Paulos, W., Astawesegn, F. H., & Gelgelu, T. B. 2019, Prevalence of diarrheal diseases and associated factors among under-five children in Dale District, Sidama zone, Southern Ethiopia: a cross-sectional study. BMC public health, 19, 1-10.
[27]. Jemal, S. 2018, Knowledge and practices of hand washing among health professionals in Dubti Referral Hospital, Dubti, Afar, Northeast Ethiopia. Advances in preventive medicine, 2018(1), 5290797.
Viewed PDF 293 36 -
 Green synthesis and Characterization of Copper oxide nanoparticles using Bauhinia tomentosa leaf extract and evaluation of its antimicrobial activity against wound pathogensAuthor: Jeevan PandiyanDOI: 10.21522/TIJPH.2013.SE.25.01.Art019
Green synthesis and Characterization of Copper oxide nanoparticles using Bauhinia tomentosa leaf extract and evaluation of its antimicrobial activity against wound pathogensAuthor: Jeevan PandiyanDOI: 10.21522/TIJPH.2013.SE.25.01.Art019Green synthesis and Characterization of Copper oxide nanoparticles using Bauhinia tomentosa leaf extract and evaluation of its antimicrobial activity against wound pathogens
Abstract:
The increasing problem of antibiotic resistance in the treatment of bacterial diseases has made the development of novel antimicrobial drugs crucial. In this study, we examine the environmentally friendly synthesis of copper oxide nanoparticles (CuONPs) using Bauhinia tomentosa (BT) leaf extract. The plant B. tomentosa, which has been used for centuries for its therapeutic uses, offers an eco-friendly and sustainable way to produce nanoparticles. Diverse techniques such as Transmission Electron Microscopy (TEM), UV-Vis spectroscopy, Scanning Electron Microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), and Energy-Dispersive X-ray spectroscopy (EDX), were used to characterize these BT-CuONPs. The synthesis of CuO NPs is confirmed through UV-Vis spectroscopy, showing an absorption peak at 225nm. Morphological analysis via SEM and TEM reveals the presence of spherical and irregularly shaped nanoparticles, typically around 50 nm in size. FTIR analysis identifies characteristic absorption bands by indication of fuctional groups from leaf extract that may interact with nanoparticles. Additionally, EDX analysis confirms the elemental composition, predominantly revealing peaks for copper and oxygen, validation successful synthesis of BT-CuONPs. BT-CuONPs were synthesized and evaluated for their antimicrobial effectiveness against wound pathogens, including multiple drug resistant organisms. The findings showed a notable antimicrobial efficacy with highest inhibition zone against P. aeruginosa at 40mm, suggesting that BT-CuONPs had strong antimicrobial potential. The promise of employing plant extracts more especially, B. tomentosa as a green synthesis route for CuONPs and their use in the fight against drug-resistant microbial infections is highlighted in this study. In the continuous fight against drug resistance, these successful production and characterization of nanoparticles highlight their potential as strong antimicrobial agents.
Green synthesis and Characterization of Copper oxide nanoparticles using Bauhinia tomentosa leaf extract and evaluation of its antimicrobial activity against wound pathogens
References:
[1]. Salam, M. A., Al-Amin, M. Y., Salam, M. T., Pawar, J. S., Akhter, N., Rabaan, A. A., Alqumber, M. A., 2023, Antimicrobial resistance: a growing serious threat for global public health. In Healthcare, 11(13), 1946.
[2]. Kumari, P., Luqman, S., Meena, A., 2019, Application of the combinatorial approaches of medicinal and aromatic plants with nanotechnology and its impacts on healthcare. DARU Journal of Pharmaceutical Sciences, 27, 475-489.
[3]. Alshammari, B. H., Lashin, M. M., Mahmood, M. A., Al-Mubaddel, F. S., Ilyas, N., Rahman, N., & Khan, R., 2023, Organic and inorganic nanomaterials: fabrication, properties and applications. RSC Advances, 13(20), 13735-13785.
[4]. Bezza, F. A., Tichapondwa, S. M., Chirwa, E. M., 2020, Fabrication of monodispersed copper oxide nanoparticles with potential application as antimicrobial agents. Scientific Reports, 10(1), 16680.
[5]. Fazal, A., Ara, S., Ishaq, M. T., Sughra, K., 2022, Green fabrication of copper oxide nanoparticles: a comparative antibacterial study against gram-positive and gram-negative bacteria. Arabian Journal for Science and Engineering, 47(1), 523-533.
[6]. Majumdar, D., Ghosh, S., 2021, Recent advancements of copperoxide based nanomaterials for supercapacitor applications. Journal of Energy Storage, 34, 101995.
[7]. Nair, G. M., Sajini, T., Mathew, B., 2022, Advanced green approaches for metal and metal oxide nanoparticles synthesis and their environmental applications. Talanta Open, 5, 100080.
[8]. Jeevanandam, J., Kiew, S. F., Boakye-Ansah, S., Lau, S. Y., Barhoum, A., Danquah, M. K., & Rodrigues, J., 2022, Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale, 14(7), 2534-2571.
[9]. Mukundan, D., Mohankumar, R., Vasanthakumari, R., 2015, Green synthesis of silver nanoparticles using leaves extract of Bauhinia tomentosa linn and its invitro anticancer potential. Materials Today: Proceedings, 2(9), 4309-4316.
[10]. Mukundan, D., Mohankumar, R., Vasanthakumari, R., 2015, Green synthesis of silver nanoparticles using leaves extract of Bauhinia tomentosa linn and its invitro anticancer potential. Materials Today: Proceedings, 2(9), 4309-4316.
[11]. Singh, A., Kaur, J., Kapoor, M., 2023, Phytochemical screening and antibacterial potential of Methanol, Ethanol and aqueous extracts from Seed, Bark and Leaf of Bauhinia tomentosa L. Agricultural Science Digest-A Research Journal, 43(1), 10-17.
[12]. Vakayil, R., Muruganantham, S., Kabeerdass, N., Rajendran, M., Ramasamy, S., Alahmadi, T. A., Mathanmohun, M., 2021, Acorus calamus-zinc oxide nanoparticle coated cotton fabrics shows antimicrobial and cytotoxic activities against skin cancer cells. Process Biochemistry, 111, 1-8.
[13]. Prabu, P., &Losetty, V., 2024, Green synthesis of copper oxide nanoparticles using MacroptiliumLathyroides (L) leaf extract and their spectroscopic characterization, biological activity and photocatalytic dye degradation study. Journal of Molecular Structure, 1301, 137404.
[14]. Balaji, T., Manushankar, C. M., Al-Ghanim, K. A., Kamaraj, C., Thirumurugan, D., Thanigaivel, S., Govindarajan, M., 2023, Padina boergesenii-mediated copper oxide nanoparticles synthesis, with their antibacterial and anticancer potential. Biomedicines, 11(8), 2285.
[15]. Kabeerdass, N., Thangasamy, S., Murugesan, K., Arumugam, N., Almansour, A. I., Suresh Kumar, R., Mathanmohun, M., 2022, Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach. Green Processing and Synthesis, 11(1), 875-885.
[16]. Abdelbaky, A. S., Abd El-Mageed, T. A., Babalghith, A. O., Selim, S., Mohamed, A. M., 2022. Green synthesis and characterization of ZnO nanoparticles using Pelargonium odoratissimum (L.) aqueous leaf extract and their antioxidant, antibacterial and anti-inflammatory activities. Antioxidants, 11(8), 1444.
[17]. Ali Alhaidrai, S. A., Al-Hadi, F. A., Al-Kaf, A. G., Taj, A. D., 2022, A. Phytochemical Screening by FTIR Spectroscopic Analysis in the Methanolic Extracts Coffee (C. Arabica. L) to Seeds and Peels (Unroasted and Roasted) Cultivars Grown in Yemen. Bioequiv. Bioavailab. Int. J 6, 000179.
[18]. Renuga, D., Jeyasundari, J., Athithan, A. S., Jacob, Y. B. A., 2020, Synthesis and characterization of copper oxide nanoparticles using Brassica oleracea var. italic extract for its antifungal application. Materials Research Express, 7(4), 045007.
[19]. Elango, B., Okram, G. S., Mathanmohun, M., 2023, Pharmacological Applications of Plant-Mediated Synthesized Nanomaterials. Current Pharmacology Reports, 9(6), 511-522.
[20]. Osman, A. I., Zhang, Y., Farghali, M., Rashwan, A. K., Eltaweil, A. S., Abd El-Monaem, E. M., Yap, P. S. 2024, Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environmental Chemistry Letters, 22(2), 841-887.
[21]. Andualem, W. W., Sabir, F. K., Mohammed, E. T., Belay, H. H., Gonfa, B. A., 2020, Synthesis of copper oxide nanoparticles using plant leaf extract of Catha edulis and its antibacterial activity. Journal of Nanotechnology, 2020(1), 2932434.
[22]. Muteeb, G., Rehman, M. T., Shahwan, M., Aatif, M., 2023, Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals, 16(11), 1615.
[23]. Renganathan, S., Subramaniyan, S., Karunanithi, N., Vasanthakumar, P., Kutzner, A., Kim, P. S., Heese, K., 2021, antibacterial, antifungal, and antioxidant activities of silver nanoparticles biosynthesized from Bauhinia Tomentosa Linn. Antioxidants, 10(12), 1959.Antioxidants 10, no. 12 (2021): 1959.
[24]. Gnanamoorthy, G., Ramar, K., Ali, D., Yadav, V. K., Kumar, G., 2022, Synthesis and effective performance of Photocatalytic and Antimicrobial activities of Bauhinia tomentosa Linn plants using of gold nanoparticles. Optical Materials, 123, 111945.
[25]. Sharmila, G., Pradeep, R. S., Sandiya, K., Santhiya, S., Muthukumaran, C., Jeyanthi, J., Thirumarimurugan, M., 2018, Biogenic synthesis of CuO nanoparticles using Bauhinia tomentosa leaves extract: characterization and its antibacterial application. Journal of Molecular Structure, 1165, 288-292.
[26]. Ramar, K., Vasanthakumar, V., Priyadharsan, A., Priya, P., Raj, V., Anbarasan, P. M., Jafar Ahamed, A., 2018, Green synthetic approach of silver nanoparticles from Bauhinia tomentosa Linn. leaves extract for potent photocatalytic and in vitro biological applications. Journal of Materials Science: Materials in Electronics, 29, 11509-11520.
[27]. Devaraji, M., Thanikachalam, P. V., & Elumalai, K., 2024, The Potential of Copper Oxide Nanoparticles in Nanomedicine: A Comprehensive Review. Biotechnology Notes.
[28]. Naz, S., Gul, A., Zia, M., Javed, R., 2023, Synthesis, biomedical applications, and toxicity of CuO nanoparticles. Applied Microbiology and Biotechnology, 107(4), 1039-1061.
[29]. Velsankar, K., Suganya, S., Muthumari, P., Mohandoss, S., Sudhahar, S., 2021, Ecofriendly green synthesis, characterization and biomedical applications of CuO nanoparticles synthesized using leaf extract of Capsicum frutescens. Journal of Environmental Chemical Engineering, 9(5), 106299.
[30]. Govindharaj, S., Nizar, A. M., Akilaa, O. B., Shanmugam, R., Thangavelu, L., 2024, Green Synthesis of Tannic Acid-Mediated Chromium Oxide Nanocomposites using Acalypha Indica and Carica Papaya Leaf and its Biomedical Applications. Nanotechnology Perceptions, 304-326.
[31]. Gayathri, B., Shanmugam, R., Thangavelu, L., Muthukumaran, D., 2024, Antibacterial Activity of Selenium Nanoparticles Synthesized using Mimosa Pudica against Wound Pathogens. Nanotechnology Perceptions, 369-376.
[32]. PunnoseKovoor, A., Krishnan, A., Manigandan, P., Shanmugam, R., Thangavelu, L., 2024, Green Synthesis of Silver Nanoparticles from Aegle Marmelos and Cissus Quadrangularis Leaf Extract and Evaluation of Its Anti-Inflammatory and Antioxidant Effect. Nanotechnology Perceptions, 276-289.
Viewed PDF 335 22 -
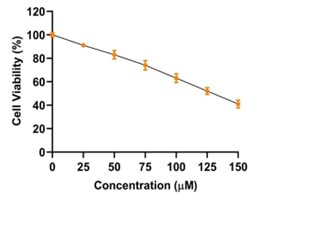 The Role of Pyrogallol as an Anti-cancer Agent Reduces Cell Proliferation in Lung Cancer Cells via AKT/PI3K Signaling Pathways - An In vitro and In silico ApproachesAuthor: R. PriyadharshiniDOI: 10.21522/TIJPH.2013.SE.25.01.Art020
The Role of Pyrogallol as an Anti-cancer Agent Reduces Cell Proliferation in Lung Cancer Cells via AKT/PI3K Signaling Pathways - An In vitro and In silico ApproachesAuthor: R. PriyadharshiniDOI: 10.21522/TIJPH.2013.SE.25.01.Art020The Role of Pyrogallol as an Anti-cancer Agent Reduces Cell Proliferation in Lung Cancer Cells via AKT/PI3K Signaling Pathways - An In vitro and In silico Approaches
Abstract:
This study investigates the anticancer potential of Pyrogallol, focusing on its effects on cell viability, cell culture, docking analysis, and anticancer activity through fold change measurements. Pyrogallol, a naturally occurring phenolic compound, exhibits significant biological activity, including anticancer properties. This research highlights its therapeutic efficacy against specific cancer cell lines. The methodology involved culturing cancer cells under controlled conditions, followed by treatment with varying concentrations of Pyrogallol. Cell viability assays were conducted using the MTT method to evaluate cytotoxicity. Additionally, molecular docking was performed to analyze the binding interactions of Pyrogallol with target proteins implicated in cancer pathways, revealing strong affinity and specificity. Results demonstrated a dose-dependent reduction in cancer cell viability, indicating Pyrogallol's cytotoxic effects. Docking analysis revealed key interactions with critical residues in cancer-related proteins, supporting its mechanistic role in inducing apoptosis. Anticancer activity, measured by fold change in gene expression, confirmed significant upregulation of pro-apoptotic markers and downregulation of survival-associated markers. The findings underline Pyrogallol's potential as an anticancer agent with promising therapeutic implications. This comprehensive approach combining In vitro assays and computational analysis provides a foundation for future studies to explore Pyrogallol's clinical applications in cancer treatment.
The Role of Pyrogallol as an Anti-cancer Agent Reduces Cell Proliferation in Lung Cancer Cells via AKT/PI3K Signaling Pathways - An In vitro and In silico Approaches
References:
[1]. Gupta, A., Jeyakumar, E., Lawrence, R., 2021, Pyrogallol: a competent therapeutic agent of the future. Biotech Env Sc, 23(2):213-7, https://www.envirobiotechjournals.com/AJMBES/v23i22021/AJ-15.pdf
[2]. Revathi, S., Hakkim, F. L., Kumar, N. R., Bakshi, H. A., Rashan, L., Al-Buloshi, M., Hasson, S. S. A. A., Krishnan, M., Javid, F., & Nagarajan, K., 2018, Induction of HT-29 Colon Cancer Cells Apoptosis by Pyrogallol with Growth Inhibiting Efficacy Against Drug-Resistant Helicobacter pylori. Anti-cancer agents in medicinal chemistry, 18(13), 1875–1884. https://doi.org/10.2174/1871520618666180806104902
[3]. Ahn, H., Im, E., Lee, D. Y., Lee, H. J., Jung, J. H., Kim, S. H., 2019, Antitumor effect of pyrogallol via miR-134 mediated S phase arrest and inhibition of PI3K/AKT/Skp2/cMyc signaling in hepatocellular carcinoma. International journal of molecular sciences, 20(16):3985, https://pubmed.ncbi.nlm.nih.gov/31426282/
[4]. Sampath, G., Shyu, D. J., Rameshkumar, N., Krishnan, M., Sivasankar, P., Kayalvizhi, N., 2019, Synthesis and characterization of pyrogallol capped silver nanoparticles and evaluation of their in vitro anti-bacterial, anti-cancer profile against AGS cells. Journal of Cluster Science, 32:549-57, https://link.springer.com/article/10.1007/s10876-020-01813-8
[5]. Revathi, S., Hakkim, F. L., Ramesh Kumar, N., Bakshi, H. A., Sangilimuthu, A. Y., Tambuwala, M. M., Changez, M., Nasef, M. M., Krishnan, M., M., M., & Kayalvizhi, N., 2019, In Vivo Anti-Cancer Potential of Pyrogallol in Murine Model of Colon Cancer. Asian Pacific journal of cancer prevention: APJCP, 20(9), 2645–2651. https://doi.org/10.31557/APJCP.2019.20.9.2645
[6]. Liu, H., Chang, Y., Jin, L., 2024, Pyrogallol Suppresses the Tumor Progression via Modulating Aerobic Glycolysis and STAT2 Signaling Pathway in Non-small Cell Lung Carcinoma. Pharmacognosy Magazine, 20(3):973-82,https://journals.sagepub.com/doi/epub/10.1177/09731296241242542
[7]. Cicek, B., Tgzd. A., Çiçek, B., et al. 2023, Pyrogallol Induces Selective Cytotoxicity in SH-SY5Y and Cortical Neuron Cells. Recent Trends in Pharmacology, 1(1):1-10, https://dergipark.org.tr/en/pub/rtpharma/issue/77156/1260696
[8]. Arjsri, P., Mapoung, S., Semmarath, W., Srisawad, K., Tuntiwechapikul, W., Yodkeeree, S., Dejkriengkraikul, P., 2023, Pyrogallol from Spirogyra neglecta Inhibits Proliferation and Promotes Apoptosis in Castration-Resistant Prostate Cancer Cells via Modulating Akt/GSK-3 β/β-catenin Signaling Pathway. International Journal of Molecular Sciences. 29;24(7):6452, https://pubmed.ncbi.nlm.nih.gov/37047425/
[9]. Yasothkumar, D., Jayaraman, S., Ramalingam, K., Ramani, P., 2023, In vitro Anti-Inflammatory and Antioxidant Activity of Seed Ethanolic Extract of Pongamia pinnata. Biomed Pharmacol J.16(4),2187-2193, https://biomedpharmajournal.org/vol16no4/in-vitro-anti-inflammatory-and-antioxidant-activity-of-seed-ethanolic-extract-of-pongamia-pinnata/
[10]. Praveen, G., Krishnamoorthy, K., Veeraraghavan, V. P., Jayaraman, S., 2024, Antioxidant and Anti-Inflammatory Activity of the Ethanolic Extract of Euphorbia Hirta Leaf Extract: An In Vitro and In Silico Study. J Pharm Bioallied Sci. 16(Suppl 2): S1304-7, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11174252/
[11]. Wu, H. Y., Yang, F. L., Li, L. H., Rao, Y. K., Ju, T. C., Wong, W. T., Hsieh, C. Y., Pivkin, M. V., Hua, K. F., & Wu, S. H. 2018, Ergosterol peroxide from marine fungus Phoma sp. induces ROS-dependent apoptosis and autophagy in human lung adenocarcinoma cells. Scientific Reports, 8(1), 17956. https://doi.org/10.1038/s41598-018-36411-2
[12]. Zuvairiya, U., Jayaraman, S., Sankaran, K., Veeraraghavan, V. P., R, G., 2024, Studies on the Effect of Piperine on Hepatocyte Nuclear Factor 1 Alpha (HNF-1α) and Sterol Regulatory Element-Binding Protein 1c (SREBP-1c) Levels in High-Fat-Diet and Sucrose-Induced Type 2 Diabetes Mellitus Rats. Cureus. 16(2): e5406, https://pubmed.ncbi.nlm.nih.gov/38481895/
[13]. Jayaraman, S., Natararaj, S., Veeraraghavan, V. P., 2024, Hesperidin Inhibits Oral Cancer Cell Growth via Apoptosis and Inflammatory Signaling-Mediated Mechanisms: Evidence From In Vitro and In Silico Analyses. Cureus. 16(2): e53458, https://pubmed.ncbi.nlm.nih.gov/38435153/
[14]. Pazhani, J., Veeraraghavan, V. P., Jayaraman, S., 2023, Molecular docking analysis of proflavin with the Wnt pathway targets for OSCC. Bioinformation, 19(4):464, https://pmc.ncbi.nlm.nih.gov/articles/PMC10563574/
[15]. Mostafa, R. G., Abd-ElHamid, E. S., El-Bolok, A. H., Eldin, E. A., Tohamy, S. M., 2021, Possible Effect of Pyrogallol on Tongue Squamous Cell Carcinoma SCC-25 Cells. Minia Journal of Medical Research, 32(2):77-87, https://mjmr.journals.ekb.eg/article_231548.html
[16]. Arjsri, P., Mapoung, S., Semmarath, W., et al. 2023, Pyrogallol from Inhibits Proliferation and Promotes Apoptosis in Castration-Resistant Prostate Cancer Cells via Modulating Akt/GSK-3/-catenin Signaling Pathway. Int J Mol Sci.24(7):6452, https://pubmed.ncbi.nlm.nih.gov/37047425/
[17]. Alavi Rafiee, S., Farhoosh, R., Sharif, A., 2018, Antioxidant activity of gallic acid as affected by an extra carboxyl group than pyrogallol in various oxidative environments. European Journal of Lipid Science and Technology, 120(11):1800319, https://onlinelibrary.wiley.com/doi/abs/10.1002/ejlt.201800319
[18]. Ghasemi, E., Bamoharram, F. F., Karimi, E., Meghdadi, P., 2024, Investigating the anticancer potential and apoptotic signaling of nanocapsule-loaded pyrogallol in ovarian cancer cells (A2780). Results in Chemistry, 11:101772, https://www.sciencedirect.com/science/article/pii/S2211715624004685
[19]. Yang, C. J., Wang, C. S., Hung, J. Y., Huang, H. W., Chia, Y. C., Wang, P. H., Weng, C. F., Huang, M. S., 2009, Pyrogallol induces G2-M arrest in human lung cancer cells and inhibits tumor growth in an animal model. Lung cancer (Amsterdam, Netherlands), 66(2), 162–168. https://doi.org/10.1016/j.lungcan.2009.01.016
Viewed PDF 216 8 -
 Harnessing the Power of Alkaloids in Breast Cancer Treatment: A Review of Therapeutic Efficacy and ChallengesAuthor: Sridevi GopathyDOI: 10.21522/TIJPH.2013.SE.25.01.Art021
Harnessing the Power of Alkaloids in Breast Cancer Treatment: A Review of Therapeutic Efficacy and ChallengesAuthor: Sridevi GopathyDOI: 10.21522/TIJPH.2013.SE.25.01.Art021Harnessing the Power of Alkaloids in Breast Cancer Treatment: A Review of Therapeutic Efficacy and Challenges
Abstract:
Cancer is a massive public health concern on a global scale. Developed nations have greater rates of breast cancer. Survival rates have increased as a result of early discovery. However, there are still major ongoing challenges which include variances in the availability of care, aggressive tumor subtypes, and the emergence of treatment resistance. These medical procedures have been linked with various adverse effects, prompting the usage of natural substances because they have less to no negative impact. Among these natural compounds is the class of alkaloids. These phytochemicals form a wide range of organic compounds that are naturally present and mostly derived from plant-kinds, but then they are also found in microbes, yeasts, and faunas. Characterized by nitrogen atoms, alkaloids exhibit more biological properties, making them of significant interest in various research fields. Alkaloids exhibit antiproliferative, antibacterial, and antioxidant properties and act as an abundant source for drug discovery and development. This study reviews the alkaloids matrine, noscapine, capsaicin, harmine, and Mahanine and describes their modes of action. These alkaloids can be utilized as tools of combination treatment and have been illustrated to initiate autophagy, reduce tumor volume, cause apoptosis, disrupt microtubule function, inhibit topoisomerase enzymes, and signaling pathway alterations involved in cell growth and survival to inhibit cell multiplication and migration. This review presents comprehensive data on the therapeutic potential of alkaloids against breast cancer.
Harnessing the Power of Alkaloids in Breast Cancer Treatment: A Review of Therapeutic Efficacy and Challenges
References:
[1] Sharma, G. N., Dave, R., Sanadya, J., Sharma, P., & Sharma, K., 2010, Various types and management of breast cancer: an overview. Journal of Advanced Pharmaceutical Technology & Research, 1(2), pp.109-126.
[2] Menezes, M. R., 2015, The Biology of Cancer. Yale J Biol Med, 88(2),199–200. PMCID: PMC4445444.
[4] Orrantia-Borunda, E., Anchondo-Nuñez, P., Acuña-Aguilar, L. E., Gómez-Valles, F. O., & Ramírez-Valdespino, C. A., 2022, Subtypes of breast cancer. Breast Cancer [Internet].
[5] Rezano, A., Ridhayanti, F., Rangkuti, A. R., Gunawan, T., Winarno, G. N. A., & Wijaya, I., 2021, Cytotoxicity of simvastatin in human breast cancer MCF-7 and MDA-MB-231 cell lines. Asian Pacific Journal of Cancer Prevention, 22(S1), 33-42.
[6] Pazhani, J., Chanthu, K., Jayaraman, S., & Varun, B. R., 2023, Evaluation of salivary MMP-9 in oral squamous cell carcinoma and oral leukoplakia using ELISA. Journal of Oral and Maxillofacial Pathology, 27(4), 649-654.
[7] Rampogu, S., Balasubramaniyam, T., & Lee, J. H., 2022, Phytotherapeutic applications of alkaloids in treating breast cancer. Biomedicine & Pharmacotherapy, 155, 113760.
[8] Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P., & Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), 704-713.
[9] Sruthi, M. A., Mani, G., Ramakrishnan, M., & Selvaraj, J., 2023, Dental caries as a source of Helicobacter pylori infection in children: An RT‐PCR study. International Journal of Paediatric Dentistry, 33(1), 82-88.
[10] Niazi, P., & Monib, A. W., 2024, The role of plants in traditional and modern medicine. Journal of Pharmacognosy and Phytochemistry, 13(2), 643-647.
[11] Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P., & Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), 1007-1013.
[12] Isah, T., 2016, Anticancer alkaloids from trees: Development into drugs. Pharmacognosy Reviews, 10(20), 90.
[13] McChesney, J. D., Venkataraman, S. K., & Henri, J. T., 2007, Plant natural products: back to the future or into extinction? Phytochemistry, 68(14), 2015-2022.
[14] Olofinsan, K., Abrahamse, H., & George, B. P., 2023, Therapeutic role of alkaloids and alkaloid derivatives in cancer management. Molecules, 28(14), 5578.
[15] Robinson, T., 1974, Metabolism and Function of Alkaloids in Plants: Alkaloids appear to be active metabolites, but their usefulness to plants remains obscure. Science, 184(4135), 430-435.
[16] Dey, P., Kundu, A., Kumar, A., Gupta, M., Lee, B. M., Bhakta, T., & Kim, H. S. 2020, Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent advances in natural products analysis (pp. 505-567). Elsevier.
[17] Liu, X. J., Cao, M. A., Li, W. H., Shen, C. S., Yan, S. Q., & Yuan, C. S., 2010, Alkaloids from Sophora flavescens Aition. Fitoterapia, 81(6), 524-527.
[18] He, X., Fang, J., Huang, L., Wang, J., & Huang, X., 2015, Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. Journal of ethnopharmacology, 172, 10-29.
[19] ur Rashid, H., Xu, Y., Muhammad, Y., Wang, L., & Jiang, J., 2019, Research advances on anticancer activities of matrine and its derivatives: An updated overview. European Journal of Medicinal Chemistry, 161, 205-238.
[20] Yu, P., Liu, Q., Liu, K., Yagasaki, K., Wu, E., & Zhang, G., 2009, Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κ B signaling. Cytotechnology, 59, 219-229.
[21] Li, H., Li, X., Bai, M., Suo, Y., Zhang, G., & Cao, X., 2015, Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via upregulation of Bax and downregulation of Bcl-2. International Journal of Clinical and Experimental Pathology, 8(11), 14793.
[22] Li, L. Q., Li, X. L., Wang, L., Du, W. J., Guo, R., Liang, H. H., & Jiang, H. C., 2012, Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cellular Physiology and Biochemistry, 30(3), 631-641.
[23] Fathima, J. S., Jayaraman, S., Sekar, R., & Syed, N. H., 2024, The role of MicroRNAs in the diagnosis and treatment of oral premalignant disorders. Odontology, 1-10.
[24] Zhou, B. G., Wei, C. S., Zhang, S., Zhang, Z., & Gao, H. M., 2018, Matrine reversed multidrug resistance of breast cancer MCF‐7/ADR cells through PI3K/AKT signaling pathway. Journal of Cellular Biochemistry, 119(5), 3885-3891.
[25] Wang, X. Y., Liang, L., Chang, J. L., Yang, M. H., & Li, Z. G., 2010, Toxicity of matrine in Kunming mice. Nan fang yi ke da xue xue bao= Journal of Southern Medical University, 30(9), 2154-2155.
[26] Sagar, S., Ramani, P., Moses, S., Gheena, S., & Selvaraj, J., 2024, Correlation of salivary cytokine IL-17A and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology, 1-10.
[27] Rampogu, S., Balasubramaniyam, T., & Lee, J. H., 2022, Phytotherapeutic applications of alkaloids in treating breast cancer. Biomedicine & Pharmacotherapy, 155, 113760.
[28] Tomar, V., Kukreti, S., Prakash, S., Madan, J., & Chandra, R., 2017, Noscapine and its analogs as chemotherapeutic agent: current updates. Current Topics in Medicinal Chemistry, 17(2), 174-188.
[29] Quisbert-Valenzuela, E. O., & Calaf, G. M., 2016, Apoptotic effect of noscapine in breast cancer cell lines. International Journal of Oncology, 48(6), 2666-2674.
[30] Sajadian, S., & Vatankhah, M., 2015, Cell cycle arrest and apoptogenic properties of opium alkaloids noscapine and papaverine on breast cancer stem cells. Toxicol Mech Methods,25 (5),388–395.
[31] Landen, J. W., Lang, R., McMahon, S. J., Rusan, N. M., Yvon, A. M., Adams, A. W., & Joshi, H. C., 2002, Noscapine alters microtubule dynamics in living cells and inhibits the progression of melanoma. Cancer Research, 62(14), 4109-4114.
[32] Singh, H., Singh, P., Kumari, K., Chandra, A., K Dass, S., & Chandra, R., 2013, A review on noscapine, and its impact on heme metabolism. Current Drug Metabolism, 14(3), 351-360.
[33] Chou, C. C., Wu, Y. C., Wang, Y. F., Chou, M. J., Kuo, S. J., & Chen, D. R., 2009, Capsaicin-induced apoptosis in human breast cancer MCF-7 cells through caspase-independent pathway. Oncology Reports, 21(3), 665-671.
[34] Wu, D., Jia, H., Zhang, Z., & Li, S., 2020, Capsaicin suppresses breast cancer cell viability by regulating the CDK8/PI3K/Akt/Wnt/β‑catenin signaling pathway. Molecular Medicine Reports, 22(6), 4868-4876.
[35] Roy, M., Chakraborty, S., Siddiqi, M., & Bhattacharya, R. K., 2002, Induction of apoptosis in tumor cells by natural phenolic compounds. Asian Pac J Cancer Prev, 3(1), 61-67.
[36] Patel, K., Gadewar, M., Tripathi, R., Prasad, S. K., & Patel, D. K., 2012, A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pacific Journal of Tropical Biomedicine, 2(8), 660-664.
[37] Ma, Y., & Wink, M., 2010, The beta‐carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, 24(1), 146-149.
[38] Ding, Y., He, J., Huang, J., Yu, T., Shi, X., Zhang, T., & Peng, C., 2019, Harmine induces anticancer activity in breast cancer cells via targeting TAZ. International Journal of Oncology, 54(6), 1995-2004.
[39] Yao, P., Yao, P., Ku, X., & Yang, J., 2023, Harmine suppresses the malignant phenotypes and PI3K activity in breast cancer. Anti-Cancer Drugs, 34(3), 373-383.
[40] Hu, Y., Yu, X., Yang, L., Xue, G., Wei, Q., Han, Z., & Chen, H., 2024, Research progress on the antitumor effects of harmine. Frontiers in Oncology, 14, 1382142.
[41] Ismail, A., Noolu, B., Gogulothu, R., Perugu, S., Rajanna, A., & Babu, S. K., 2016, Cytotoxicity and proteasome inhibition by alkaloid extract from Murraya koenigii leaves in breast cancer cells—molecular docking studies. Journal of Medicinal Food, 19(12), 1155-1165.
[42] Wada, M., Kosaka, M., Saito, S., Sano, T., Tanaka, K., & Ichihara, A., 1993, Serum concentration and localization in tumor cells of proteasomes in patients with hematologic malignancy and their pathophysiologic significance. The Journal of Laboratory and Clinical Medicine, 121(2), 215-223.
[43] Noolu, B., & Ismail, A., 2015, Anti-proliferative and proteasome inhibitory activity of Murraya koenigii leaf extract in human cancer cell lines. Discovery Phytomedicine-Journal of Natural Products Research and Ethnopharmacology, 2(1), 1-9.
[44] Das, R., Bhattacharya, K., Sarkar, S., Samanta, S. K., Pal, B. C., & Mandal, C., 2014, RETRACTED ARTICLE: Mahanine synergistically enhances cytotoxicity of 5-fluorouracil through ROS-mediated activation of PTEN and p53/p73 in colon carcinoma. Apoptosis, 19(1), 149-164.
[45] Das, M., Kandimalla, R., Gogoi, B., Dutta, K. N., Choudhury, P., Devi, R., & Samanta, S. K. (2019). Mahanine, A dietary phytochemical, represses mammary tumor burden in rat and inhibits subtype regardless breast cancer progression through suppressing self-renewal of breast cancer stem cells. Pharmacological Research, 146, 104330.
[46] Samanta, S. K., Lee, J., Hahm, E. R., & Singh, S. V., 2018, Peptidyl‐prolyl cis/trans isomerase Pin1 regulates withaferin A‐mediated cell cycle arrest in human breast cancer cells. Molecular Carcinogenesis, 57(7), 936-946.
[47] Du, Z., Tong, X., & Ye, X., 2013, Cyclin D1 promotes cell cycle progression through enhancing NDR1/2 kinase activity independent of cyclin-dependent kinase 4. Journal of Biological Chemistry, 288(37), 26678-26687.
[48] Kim, S. H., & Singh, S. V., 2014, Mammary cancer chemoprevention by withaferin A is accompanied by in vivo suppression of self-renewal of cancer stem cells. Cancer Prevention Research, 7(7), 738-747.
[49] Yasothkumar, D., Ramani, P., Jayaraman, S., Ramalingam, K., & Tilakaratne, W. M., 2024, Expression Profile of Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck Pathology, 18(1), 28.
Viewed PDF 198 28 -
 Phytochemical Analysis of Trikatu (Thirukadugam): A Study through Gas Chromatography and Mass SpectrometryAuthor: Glad MoheshDOI: 10.21522/TIJPH.2013.SE.25.01.Art022
Phytochemical Analysis of Trikatu (Thirukadugam): A Study through Gas Chromatography and Mass SpectrometryAuthor: Glad MoheshDOI: 10.21522/TIJPH.2013.SE.25.01.Art022Phytochemical Analysis of Trikatu (Thirukadugam): A Study through Gas Chromatography and Mass Spectrometry
Abstract:
Trikatu (Thirukadugam) is an amalgamation of various herbal parts which is using in traditional medicinal system like siddha, Ayurvedha, Homeopathy etc. This formulation is effective in respiratory problem acts as bronchodilator and expectorants. It enhances the digestive juice secretion and increase the appetite. Antiobesity property regulate the serum cholesterol and triglycerides level. Hence it might increase sensitivity of insulin receptor and its phytochemical component acts as bio enhancers to promote antidiabetic properties. It is utilized to treat the arthritis, gout disease and good detoxifier agent. Although preceding report shows Trikatu has potential for therapies, phytochemical component is not well determined. This evokes us to initiate the present study, the ethanolic extract of Trikatu is subjected to phytochemical screening the following compounds like alkaloids, sterols, glycosides, saponins, carbohydrates, flavonoids, tannins and proteins has been present. Bioactive components were identified specifically, Amoxapine, 4,2'-Dihydroxychalcone,Succinic acid, Glycosides, 2,5-Cyclohexadiene-1,4-dione, 2,5-bis(1,1-dimethylpropyl)-, Synephrine, Sotalol, Dyphylline, Amrinone and Benzene, 3-[3-iodo-2-(iodomethyl)-2-methylpropyl]-1,2,4,5-tetramethyl—. These components might establish pharmacological nature and therapeutic potentials of the herbal extract.
Phytochemical Analysis of Trikatu (Thirukadugam): A Study through Gas Chromatography and Mass Spectrometry
References:
[1]. Gomathi, D., Kalaiselvi, M., Ravikumar, G., Devaki, K., & Uma, C., 2015, GC-MS analysis of bioactive compounds from the whole plant ethanolic extract of Evolvulus alsinoides (L.) L. Journal of Food Science and Technology, 52, 1212-1217.
[2]. Sam, S., 2019, Importance and effectiveness of herbal medicines. Journal of Pharmacognosy and Phytochemistry, 8(2), 354-357.
[3]. Pazhani, J., Chanthu, K., Jayaraman, S., & Varun, B. R., 2023, Evaluation of salivary MMP-9 in oral squamous cell carcinoma and oral leukoplakia using ELISA. Journal of Oral and Maxillofacial Pathology, 27(4), 649-654.
[4]. Shekhar, S., Ramani, A., & Sonwaniya, R., 2023, A review on formulation and evaluation of digestive churna.
[5]. Sharma, V., Hem, K., Singh, N., & Gautam, D. N., 2015, Phytochemistry and pharmacology of trikatu. Indian Journal of Agriculture and Allied Sciences, 1(4), 193-197.
[6]. Kumar, S. S., & Mishra, S. H., 2004, Hepatoprotective activity of the Trikatu Churna-an Ayurvedic formulation. Indian Journal of Pharmaceutical Sciences, 66(3), 365-367.
[7]. Ali, B. H., Blunden, G., Tanira, M. O., & Nemmar, A., 2008, Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food and Chemical Toxicology, 46(2), 409-420.
[8]. Sagar, S., Ramani, P., Moses, S., Gheena, S., & Selvaraj, J., 2024, Correlation of salivary cytokine IL-17A and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology, 1-10.
[9]. Vijayakumar, R. S., Surya, D., Senthilkumar, R., & Nalini, N., 2002, Hypolipidemic effect of black pepper (Piper nigrum Linn.) in rats fed high fat diet. Journal of Clinical Biochemistry and Nutrition, 32, 31-42.
[10]. Panda, S., & Kar, A., 2003, Piperine lowers the serum concentrations of thyroid hormones, glucose and hepatic 5′ D activity in adult male mice. Hormone and Metabolic Research, 35(09), 523-526.
[11]. Subramaniam, K., Subramanian, S. K., Bhargav, S., Parameswari, R., Praveena, R., Ravikumar, R.,& Kumar, V. M., 2021, Review on potential antiviral and immunomodulatory properties of Piper Longum. in IOP Conference Series: Materials Science and Engineering (Vol. 1145, No. 1, p. 012099). IOP Publishing.
[12]. Biswas, P., Ghorai, M., Mishra, T., Gopalakrishnan, A. V., Roy, D., Mane, A. B., & Dey, A., 2022, Piper longum L.: A comprehensive review on traditional uses, phytochemistry, pharmacology, and health‐promoting activities. Phytotherapy Research, 36(12), 4425-4476.
[13]. Krishnan, R. P., Pandiar, D., Ramani, P., & Jayaraman, S., 2025, Molecular profiling of oral epithelial dysplasia and oral squamous cell carcinoma using next generation sequencing. Journal of Stomatology, Oral and Maxillofacial Surgery, 126(4), 102120.
[14]. Offei-Oknye, R., Patterson, J., Walker, L. T., & Verghese, M., 2015, Processing effects on phytochemical content and antioxidative potential of ginger Zingiber officale. Food and Nutrition Sciences, 6(5), 445-451.
[15]. Atal, S., Agrawal, R. P., Vyas, S., Phadnis, P., & Rai, N., 2012, Evaluation of the effect of piperine per se on blood glucose level in alloxan-induced diabetic mice. Acta Pol Pharm, 69(5), 965-969.
[16]. Kumar, B., Tiwari, S., Bajpai, V., & Singh, B., 2020, Phytochemistry of plants of genus piper. CRC Press.
[17]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P., & Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), 704-713.
[18]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P., & Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), 1007-1013.
[19]. Sruthi, M. A., Mani, G., Ramakrishnan, M., & Selvaraj, J., 2023, Dental caries as a source of Helicobacter pylori infection in children: An RT‐PCR study. International Journal of Paediatric Dentistry, 33(1), 82-88.
[20]. Ban, T. A., Wilson, W. H., & McEvoy, J. P., 1980, Amoxapine: A review of literature. International Pharmacopsychiatry, 15(3), 166-170.
[21]. Yasothkumar, D., Ramani, P., Jayaraman, S., Ramalingam, K., & Tilakaratne, W. M., 2024, Expression Profile of Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck Pathology, 18(1), 28.
[22]. Haddad, A. Q., Fleshner, N., Nelson, C., Saour, B., Musquera, M., Venkateswaran, V., & Klotz, L., 2010, Antiproliferative mechanisms of the flavonoids 2, 2′-dihydroxychalcone and fisetin in human prostate cancer cells. Nutrition and Cancer, 62(5), 668-681.
[23]. Kaur, M., & Kaushal, R., 2023, Spectroscopic investigations, ab-initio DFT calculations, molecular docking and in-vitro assay studies of novel oxovanadium (V) chalcone complexes as potential antidiabetic agents. Journal of Molecular Structure, 1271, 133994.
[24]. Saravanan, R., & Pari, L., 2006, Effect of a novel insulinotropic agent, succinic acid monoethyl ester, on lipids and lipoproteins levels in rats with streptozotocin-nicotinamide-induced type 2 diabetes. Journal of Biosciences, 31(5), 581-587.
[25]. Lattibeaudiere, K. G., & Alexander-Lindo, R. L., 2022, Oleic Acid and Succinic Acid Synergistically Mitigate Symptoms of Type 2 Diabetes in Streptozotocin‐Induced Diabetic Rats. International Journal of Endocrinology, 2022(1), 8744964.
[26]. Asong, J. A., Amoo, S. O., McGaw, L. J., Nkadimeng, S. M., Aremu, A. O., & Otang-Mbeng, W., 2019, Antimicrobial activity, antioxidant potential, cytotoxicity and phytochemical profiling of four plants locally used against skin diseases. Plants, 8(9), 350.
[27]. Fathima, J. S., Jayaraman, S., Sekar, R., & Syed, N. H., 2024, The role of MicroRNAs in the diagnosis and treatment of oral premalignant disorders. Odontology, 1-10.
[28]. Hong, N. Y., Cui, Z. G., Kang, H. K., Lee, D. H., Lee, Y. K., & Park, D. B., 2012, p-Synephrine stimulates glucose consumption via AMPK in L6 skeletal muscle cells. Biochemical and Biophysical Research Communications, 418(4), 720-724.
[29]. Cui, Z., Lee, Y., Lee, Y., & Park, D., 2015, p-Synephrine suppresses glucose production but not lipid accumulation in H4IIE liver cells. Journal of Medicinal Food, 18(1), 76-82.
[30]. Kerin, N. Z., & Jacob, S., 2011, The efficacy of sotalol in preventing postoperative atrial fibrillation: A meta-analysis. The American Journal of Medicine, 124(9), 875-e1.
[31]. Hirshman, C. A., Lawyer, C., Downes, H., Farbood, A., Rodgers, R., & Gerber, N., 1981, Dyphylline aerosol attenuates antigen-induced bronchoconstriction in experimental canine asthma. Chest, 79(4), 454-458.
[32]. Mancini, D., LeJemtel, T., & Sonnenblick, E., 1985, Intravenous use of amrinone for the treatment of the failing heart. The American Journal of Cardiology, 56(3), B8-B15.
[33]. Bayomi, S. M., Moustafa, M. A., Maarouf, A. R., & Abutaleb, M. H., 2016, Design, synthesis, biological activity and molecular modeling of new heterocyclic tetrazole derivatives. J Am Sci, 12(1), 40-56
Viewed PDF 213 4 -
 Harnessing Phytomedicines: Anticancer Strategies Against Osteosarcoma- A ReviewAuthor: Sridevi GopathyDOI: 10.21522/TIJPH.2013.SE.25.01.Art023
Harnessing Phytomedicines: Anticancer Strategies Against Osteosarcoma- A ReviewAuthor: Sridevi GopathyDOI: 10.21522/TIJPH.2013.SE.25.01.Art023Harnessing Phytomedicines: Anticancer Strategies Against Osteosarcoma- A Review
Abstract:
Osteosarcoma (OS), the most common primary bone malignancy, predominantly affects children and adolescents, with poor prognosis in advanced or metastatic cases. Originating from osteoblasts, OS is characterized by rapid proliferation, local invasion, and a high propensity for lung metastasis. It is classified as primary (central or surface) or secondary when arising from preexisting conditions. Despite advances in chemotherapy and surgery, the long-term survival rate for patients with metastatic or recurrent OS remains poor, emphasizing the need for novel therapeutic approaches. Phytomedicine, derived from plant-based compounds, has garnered attention for its potential in targeting OS molecular pathways. Phytochemicals such as curcumin, resveratrol, and epigallocatechin gallate (EGCG) exhibit therapeutic effects by modulating key pathways, including Wnt/β-catenin, PI3K/AKT/mTOR, and MAPK/ERK, which are crucial for OS cell migration, proliferation, and survival. These compounds inhibit angiogenesis, promote apoptosis, and reduce metastasis by regulating the epithelial-to-mesenchymal transition (EMT). Additionally, they induce reactive oxygen species (ROS), trigger autophagy, and disrupt cellular signaling, effectively killing OS cells. Emerging studies highlight the potential of phytomedicines to enhance current treatments and improve patient outcomes by offering less harmful and more effective options. This review explores the molecular mechanisms underlying OS and evaluates phytomedicine's role in developing innovative therapies. By integrating genetic, molecular, and clinical profiles, these findings provide valuable insights for advancing OS diagnosis and management, offering hope for more sustainable and effective treatment strategies.
Harnessing Phytomedicines: Anticancer Strategies Against Osteosarcoma- A Review
References:
[1]. Malawer, M., Sugarbaker, P. H., Shmookler, B., Bickels, J., Jelinek, J., Sugarbaker, P., & Malawer, M., 2001, Bone and soft-tissue sarcomas: epidemiology, radiology, pathology and fundamentals of surgical treatment. Musculoskeletal Cancer Surgery: Treatment of Sarcomas and Allied Diseases, 3-35.
[2]. DeVita, V., Lawrence, T., Rosenberg, S., Weinberg, R. A., & DePinho, R. A. 2008, Cancer: Principles and Practice of Oncology Vols 1 and 2. Philadelphia, PA, USA: Lippincott Williams & Wilkins.
[3]. Kantarjian, H., Wolff, R. A., & Koller, C. A. 2011, The MD Anderson manual of medical oncology.
[4]. Alessio, B., Massimiliano, D. P., & Nikolin, A., 2014, Osteosarcoma symptoms, diagnosis and treatment options. Edwin Choy. Library of congress cataloging-in-publication data.
[5]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P., & Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal Of Oral Biology And Craniofacial Research, 13(6), 704–713. https://doi.org/10.1016/j.jobcr.2023.09.00.
[6]. Jame, A., James, L., Gulley., Carmen, J., 2014, Allegra. Bethesda handbook of clinical oncology - Fourth edition © 2014 by Lippincott Williams & Wilkins, a Wolters Kluwer business. Section 7: Musculoskeletal; 21. Sarcomas and Malignancies of the Bone, Patrick J. Mansky and Lee J. Helman. ISBN 978-1-4511-8758-8. Library of Congress Cataloging-in-Publication Data.
[7]. Kazantseva, L., Becerra, J., & Santos-Ruiz, L., 2022, Traditional Medicinal Plants as a Source of Inspiration for Osteosarcoma Therapy. Molecules (Basel, Switzerland), 27(15),5008. https://doi.org/10.3390/molecules27155008
[8]. Durfee, R. A., Mohammed, M., & Luu, H. H. 2016, Review of Osteosarcoma and Current Management. Rheumatology and therapy, 3(2), 221–243. https://doi.org/10.1007/s40744-016-0046-y
[9]. Hansen, M. F., Seton, M., & Merchant, A., 2006, Osteosarcoma in Paget's disease of bone. Journal of bone and mineral research: The official journal of the American Society for Bone and Mineral Research, 21 Suppl 2, P58–P63. https://doi.org/10.1359/jbmr.06s211.
[10]. Deyrup, A. T., Montag, A. G., Inwards, C. Y., Xu, Z., Swee, R. G., & Krishnan Unni, K., 2007, Sarcomas arising in Paget disease of bone: a clinicopathologic analysis of 70 cases. Archives of pathology & laboratory medicine, 131(6), 942–946. https://doi.org/10.5858/2007-131-942-SAIPDO.
[11]. Rickel, K., Fang, F., & Tao, J., 2017, Molecular genetics of osteosarcoma. Bone, 102, 69–79. https://doi.org/10.1016/j.bone.2016.10.017.
[12]. Fiedorowicz, M., Bartnik, E., Sobczuk, P., Teterycz, P., & Czarnecka, A. M., 2018, Molecular biology of sarcoma. Oncology in Clinical Practice, 14(6), 307-330.
[13]. Czarnecka, A. M., Synoradzki, K., Firlej, W., Bartnik, E., Sobczuk, P., Fiedorowicz, M., . & Rutkowski, P., 2020, Molecular biology of osteosarcoma. Cancers, 12(8), 2130.
[14]. Fathima, J. S., Jayaraman, S., Sekar, R., & Syed, N. H., 2024, The role of MicroRNAs in the diagnosis and treatment of oral premalignant disorders. Odontology, 1-10.
[15]. Savage, S. A., & Mirabello, L., 2011, Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma, 2011(1), 548151.
[16]. Huvos, A. G., 1986, Osteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older than 60 years. Cancer, 57(7), 1442-1449.
[17]. Rani, A. S., & Kumar, S., 1992, Transformation of non-tumorigenic osteoblast-like human osteosarcoma cells by hexavalent chromates: alteration of morphology, induction of anchorage-independence and proteolytic function. Carcinogenesis, 13(11), 2021-2027.
[18]. Dutra, F. R., & Largent, E. J., 1950, Osteosarcoma induced by beryllium oxide. The American journal of pathology, 26(2), 197.
[19]. Mazabraud, A., 1975, Experimental production of bone sarcomas in the rabbit by a single local injection of beryllium. Bulletin Du Cancer, 62(1), 49-58.
[20]. Casali, P. G., Bielack, S., Abecassis, N., Aro, H. T., Bauer, S., Biagini, R., & Blay, J. Y., 2018, Bone sarcomas: ESMO–PaedCan–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 29, iv79-iv95.
[21]. Sruthi, M. A., Mani, G., Ramakrishnan, M., & Selvaraj, J., 2023, Dental caries as a source of Helicobacter pylori infection in children: An RT‐PCR study. International Journal of Paediatric Dentistry, 33(1), 82-88.
[22]. Rothzerg, E., Xu, J., & Wood, D, 2023, Different Subtypes of Osteosarcoma: Histopathological Patterns and Clinical Behaviour. Journal of Molecular Pathology, 4(2), 99-108.
[23]. Kundu, Z. S., 2014, Classification, imaging, biopsy and staging of osteosarcoma. Indian Journal Of Orthopaedics, 48(3), 238-246.
[24]. Abate, M. E., Longhi, A., Galletti, S., Ferrari, S., & Bacci, G., 2010, Non‐metastatic osteosarcoma of the extremities in children aged 5 years or younger. Pediatric Blood & Cancer, 55(4), 652-654.
[25]. Kager, L., Zoubek, A., Pötschger, U., Kastner, U., Flege, S., Kempf-Bielack, B., & Bielack, S. S., 2003, Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. Journal of Clinical Oncology, 21(10), 2011-2018.
[26]. Pakos, E. E., Nearchou, A. D., Grimer, R. J., Koumoullis, H. D., Abudu, A., Bramer, J. A., & Ioannidis, J. P., 2009, Prognostic factors and outcomes for osteosarcoma: an international collaboration. European Journal Of Cancer, 45(13), 2367-2375.
[27]. Cho, W. H., Song, W. S., Jeon, D. G., Kong, C. B., Kim, M. S., Lee, J. A., & Lee, S. Y., 2010, Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Annals Of Surgical Oncology, 17, 702-708.
[28]. Kaste, S. C., Liu, T., Billups, C. A., Daw, N. C., Pratt, C. B., & Meyer, W. H., 2004, Tumor size as a predictor of outcome in pediatric non‐metastatic osteosarcoma of the extremity. Pediatric Blood & Cancer, 43(7), 723-728.
[29]. Kager, L., Zoubek, A., Dominkus, M., Lang, S., Bodmer, N., Jundt, G., & COSS Study Group., 2010, Osteosarcoma in very young children: experience of the Cooperative Osteosarcoma Study Group. Cancer, 116(22), 5316-5324.
[30]. Hayden, J. B., & Hoang, B. H., 2006, Osteosarcoma: basic science and clinical implications. Orthopedic Clinics, 37(1), 1-7.
[31]. Misaghi, A., Goldin, A., Awad, M., & Kulidjian, A. A., 2018, Osteosarcoma: A comprehensive review. Sicot-j, 4.
[32]. Ries, L. A. G., Melbert, D., Krapcho, M., Stinchcomb, D. G., Howlader, N., Horner, M. J., & Edwards, B., 2008, SEER cancer statistics review, 1975–2005. Bethesda, MD: National Cancer Institute, 2999.
[33]. Ajani, U. A., 2007, United States cancer statistics: 2004 incidence and mortality.
[34]. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 2001 Incidence and Mortality. Atlanta, GA: Centers for Disease Control and Prevention and National Cancer Institute; 2004.
[35]. Mirabello, L., Troisi, R. J., & Savage, S. A., 2009, International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. International Journal Of Cancer, 125(1), 229-234.
[36]. Colina, M., La Corte, R., De Leonardis, F., & Trotta, F., 2008, Paget’s disease of bone: a review. Rheumatology International, 28(11), 1069-1075.
[37]. Cooper, C., Harvey, N. C., Dennison, E. M., & van Staa, T. P., 2006, Update on the epidemiology of Paget's disease of bone. Journal of Bone and Mineral Research, 21(S2), P3-P8.
[38]. Mirabello, L., Troisi, R. J., & Savage, S. A., 2009, Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer: Interdisciplinary International Journal of the American Cancer Society, 115(7), 1531-1543.
[39]. Ries, L. A. G., Smith, M. A., Gurney, J., Linet, M., Tamra, T., Young, J., 1999, Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995.
[40]. Polednak, A. P., 1985, Primary bone cancer incidence in black and white residents of New York State. Cancer, 55(12), 2883-2888.
[41]. Parkin, D. M., Stiller, C. A., Draper, G. J., & Bieber, C. A., 1988, The international incidence of childhood cancer. International Journal of Cancer, 42(4), 511-520.
[42]. Linabery, A. M., & Ross, J. A., 2008, Trends in childhood cancer incidence in the US (1992–2004). Cancer: Interdisciplinary International Journal of the American Cancer Society, 112(2), 416-432.
[43]. Oyemade, G. A. A., & Abioye, A. A., 1982, Primary malignant tumors of bone: incidence in Ibadan, Nigeria. Journal of the National Medical Association, 74(1), 65.
[44]. Oboirien, M., & Khalid, A., 2013, Knowledge and belief about traditional bone setters’ practices in Sokoto, North-West Nigeria. Internet J Orthop Surg, 21(2).
[45]. Fraumeni JR, J. F., 1967, Stature and malignant tumors of bone in childhood and adolescence. Cancer, 20(6), 967-973.
[46]. Ruza, E., Sotillo, E., Sierrasesúmaga, L., Azcona, C., & Patiño-García, A., 2003, Analysis of polymorphisms of the vitamin D receptor, estrogen receptor, and collagen Iα1 genes and their relationship with height in children with bone cancer. Journal of pediatric hematology/oncology, 25(10), 780-786.
[47]. Spjut, H. J., 1971, Tumors of bone and cartilage. US Department of Defense, Armed Forces Institute of Pathology.
[48]. Longhi, A., Pasini, A., Cicognani, A., Baronio, F., Pellacani, A., Baldini, N., & Bacci, G., 2005, Height as a risk factor for osteosarcoma. Journal of pediatric hematology/oncology, 27(6), 314-318.
[49]. Goodman, M. A., McMaster, J. H., Drash, A. L., Diamond, P. E., Kappakas, G. S., & Scranton Jr, P. E., 1978, Metabolic and endocrine alterations in osteosarcoma patients. Cancer, 42(2), 603-610.
[50]. Mirabello, L., Pfeiffer, R., Murphy, G., Daw, N. C., Patiño-Garcia, A., Troisi, R. J., . & Savage, S. A., 2011, Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes & Control, 22, 899-908.
[51]. Matsunaga, E., 1980, Hereditary retinoblastoma: host resistance and second primary tumors. Journal of the National Cancer Institute, 65(1), 47-51.
[52]. Draper, G. J., Sanders, B. M., & Kingston, J. E., 1986, Second primary neoplasms in patients with retinoblastoma. British journal of cancer, 53(5), 661-671.
[53]. Matsunaga, E., 1980, Hereditary retinoblastoma: host resistance and second primary tumors. Journal of the National Cancer Institute, 65(1), 47-51.
[54]. Draper, G., Sanders, B., Kingston, J., 1986, Second primary neoplasms in patients with retinoblastoma. British Journal of Cancer, 53(5), 661.
[55]. Wang, L. L., Gannavarapu, A., Kozinetz, C. A., Levy, M. L., Lewis, R. A., Chintagumpala, M. M., Ruiz-Maldanado, R., Contreras-Ruiz, J., Cunniff, C., Erickson, R. P., Lev, D., Rogers, M., Zackai, E. H., & Plon, S. E., 2003, Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund-Thomson syndrome. Journal of the National Cancer Institute, 95(9), 669–674. https://doi.org/10.1093/jnci/95.9.669.
[56]. Hicks, M. J., Roth, J. R., Kozinetz, C. A., & Wang, L. L., 2007, Clinicopathologic features of osteosarcoma in patients with Rothmund-Thomson syndrome. Journal of clinical oncology : Official journal of the American Society of Clinical Oncology, 25(4), 370–375. https://doi.org/10.1200/JCO.2006.08.4558.
[57]. Porter, D. E., Holden, S. T., Steel, C. M., Cohen, B. B., Wallace, M. R., & Reid, R., 1992, A significant proportion of patients with osteosarcoma may belong to Li-Fraumeni cancer families. The Journal of bone and joint surgery. British volume, 74(6), 883–886. https://doi.org/10.1302/0301-620X.74B6.1447251.
[58]. Araki, N., Uchida, A., Kimura, T., Yoshikawa, H., Aoki, Y., Ueda, T., Takai, S., Miki, T., & Ono, K., 1991, Involvement of the retinoblastoma gene in primary osteosarcomas and other bone and soft-tissue tumors. Clinical orthopaedics and related research, (270), 271–277.
[59]. Thomas, L., Mautner, V. F., Cooper, D. N., & Upadhyaya, M., 2012, Molecular heterogeneity in malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1. Human genomics, 6(1), 18. https://doi.org/10.1186/1479-7364-6-18.
[60]. Deshpande, A., & Hinds, P. W., 2006, The retinoblastoma protein in osteoblast differentiation and osteosarcoma. Current molecular medicine, 6(7), 809–817. https://doi.org/10.2174/156652401060607080.
[61]. Feugeas, O., Guriec, N., Babin-Boilletot, A., Marcellin, L., Simon, P., Babin, S., Thyss, A., Hofman, P., Terrier, P., Kalifa, C., Brunat-Mentigny, M., Patricot, L. M., & Oberling, F., 1996, Loss of heterozygosity of the RB gene is a poor prognostic factor in patients with osteosarcoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 14(2), 467–472. https://doi.org/10.1200/JCO.1996.14.2.467.
[62]. Miller, C. W., Aslo, A., Tsay, C., Slamon, D., Ishizaki, K., Toguchida, J., Yamamuro, T., Lampkin, B., & Koeffler, H. P., 1990, Frequency and structure of p53 rearrangements in human osteosarcoma. Cancer research, 50(24), 7950–7954.
[63]. Miller, C. W., Aslo, A., Won, A., Tan, M., Lampkin, B., & Koeffler, H. P., 1996, Alterations of the p53, Rb and MDM2 genes in osteosarcoma. Journal of cancer research and clinical oncology, 122(9), 559–565. https://doi.org/10.1007/BF01213553.
[64]. Gokgoz, N., Wunder, J. S., Mousses, S., Eskandarian, S., Bell, R. S., & Andrulis, I. L., 2001, Comparison of p53 mutations in patients with localized osteosarcoma and metastatic osteosarcoma. Cancer, 92(8), 2181–2189. https://doi.org/10.1002/1097-0142(20011015)92:8<2181::aid-cncr1561>3.0.co;2-3.
[65]. Berman, S. D., Calo, E., Landman, A. S., Danielian, P. S., Miller, E. S., West, J. C., Fonhoue, B. D., Caron, A., Bronson, R., Bouxsein, M. L., Mukherjee, S., & Lees, J. A., 2008, Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proceedings of the National Academy of Sciences of the United States of America, 105(33), 11851–11856. https://doi.org/10.1073/pnas.0805462105.
[66]. Walkley, C. R., Qudsi, R., Sankaran, V. G., Perry, J. A., Gostissa, M., Roth, S. I., Rodda, S. J., Snay, E., Dunning, P., Fahey, F. H., Alt, F. W., McMahon, A. P., & Orkin, S. H., 2008, Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes & development, 22(12), 1662–1676. https://doi.org/10.1101/gad.1656808.
[67]. Desai, A. G., Qazi, G. N., Ganju, R. K., El-Tamer, M., Singh, J., Saxena, A. K., Bedi, Y. S., Taneja, S. C., & Bhat, H. K., 2008, Medicinal plants and cancer chemoprevention. Current drug metabolism, 9(7), 581–591. https://doi.org/10.2174/138920008785821657.
[68]. Yin, S. Y., Wei, W. C., Jian, F. Y., & Yang, N. S., 2013, Therapeutic applications of herbal medicines for cancer patients. Evidence-based complementary and alternative medicine : eCAM, 302426. https://doi.org/10.1155/2013/302426.
[69]. Cragg, G. M., & Newman, D. J., 2005, Plants as a source of anti-cancer agents. Journal of ethnopharmacology, 100(1-2), 72–79. https://doi.org/10.1016/j.jep.2005.05.011.
[70]. Krishnan, R. P., Pandiar, D., Ramani, P., & Jayaraman, S., 2024, Molecular profiling of oral epithelial dysplasia and oral squamous cell carcinoma using next generation sequencing. Journal of stomatology, oral and maxillofacial surgery, 126(4), 102120. Advance online publication. https://doi.org/10.1016/j.jormas.2024.102120.
[71]. Prasanth, N. V., Dilip, C., Sanal Dev, K. T., Augustine, L., & Saraswathi, R., 2010, Evaluation of in vitro cytotoxic and antioxidant activities of Ipomoea batatas. Int J Pharm Pharm Sci, 2(3), 91-2.
[72]. Umadevi, M., Kumar, K. S., Bhowmik, D., & Duraivel, S., 2013, Traditionally used anticancer herbs in India. Journal of Medicinal Plants Studies, 1(3), 56-74.
[73]. Rahmani, A. H., Alzohairy, M. A., Khan, M. A., & Aly, S. M., 2014, Therapeutic implications of black seed and its constituent thymoquinone in the prevention of cancer through inactivation and activation of molecular pathways. Evidence‐Based Complementary and Alternative Medicine, 2014(1), 724658.
[74]. Huang, W. Y., Cai, Y. Z., & Zhang, Y., 2009, Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutrition and cancer, 62(1), 1-20.
[75]. Sagar, S., Ramani, P., Moses, S., Gheena, S., & Selvaraj, J., 2024, Correlation of salivary cytokine IL-17A and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology, 1-10.
[76]. Chen, S., Jin, Z., Dai, L., Wu, H., 2018, Aloperine induces apoptosis and inhibits invasion in MG-63 and U2OS human osteosarcoma cells. Biomed Pharmacother, 97, 45-52.
[77]. Chen, C. Z., 2016, Berberine induced apoptosis of human osteosarcoma cells by inhibiting phosphoinositide 3 kinase/protein kinase B (PI3K/Akt) signal pathway activation.
[78]. Chen, G., Xia, H., Zhang, Z. G., & Yu, H. L., 2017, Resveratrol in management of bone and spinal cancers. Natural product research, 33(4), 516-526.
[79]. Wang, Y., Xu, S., Wu, Y., & Zhang, J., 2016, Cucurbitacin E inhibits osteosarcoma cells proliferation and invasion through attenuation of PI3K/AKT/mTOR signalling pathway. Bioscience reports, 36(6), e00405.
[80]. Peng, L., Liu, A., Shen, Y., Xu, H. Z., Yang, S. Z., Ying, X. Z., . & Shen, W. D., 2013, Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncology reports, 29(2), 571-578.
[81]. Li, X., Zhao, Y., Wu, W. K., Liu, S., Cui, M., & Lou, H., 2011, Solamargine induces apoptosis associated with p53 transcription-dependent and transcription-independent pathways in human osteosarcoma U2OS cells. Life sciences, 88(7-8), 314-321.
[82]. Jin, S., Xu, H. G., Shen, J. N., Chen, X. W., Wang, H., & Zhou, J. G., 2009, Apoptotic effects of curcumin on human osteosarcoma U2OS cells. Orthopaedic surgery, 1(2), 144-152.
[83]. Lv, T. Z., & Wang, G. S., 2015, Antiproliferation potential of withaferin A on human osteosarcoma cells via the inhibition of G2/M checkpoint proteins. Experimental and therapeutic medicine, 10(1), 323-329.
[84]. Pazhani, J., Chanthu, K., Jayaraman, S., & Varun, B. R., 2023, Evaluation of salivary MMP-9 in oral squamous cell carcinoma and oral leukoplakia using ELISA. Journal of Oral and Maxillofacial Pathology, 27(4), 649-654.
[85]. Chen, X. J., Duan, F. D., Zhang, H. H., Xiong, Y., & Wang, J., 2012, Sodium selenite-in duced apoptosis mediated by ROS attack in human osteosarcoma U2OS cells. Biological trace element research, 145, 1-9.
[86]. Yang, Q., Li, S., Fu, Z., Lin, B., Zhou, Z., Wang, Z., . & Cai, Z., 2017, Shikonin promotes adriamycin‑induced apoptosis by upregulating caspase‑3 and caspase‑8 in osteosarcoma. Molecular medicine reports, 16(2), 1347-1352.
[87]. Pichaiyan, V., Mariyappan, S., Saravanan, R., & Ramalingam, S., 2018, Herbal remedy for osteosarcoma-challenging evolution. Asian Journal of Pharmaceutical and Clinical Research, 52-56.
[88]. Yang, J., Nie, J., Ma, X., Wei, Y., Peng, Y., & Wei, X., 2019, Targeting PI3K in cancer: mechanisms and advances in clinical trials. Molecular cancer, 18(1), 26.
[89]. Arafeh, R., & Samuels, Y., 2019, PIK3CA in cancer: The past 30 years. In Seminars in cancer biology (Vol. 59, pp. 36-49). Academic Press.
[90]. Whitman, M., Downes, C. P., Keeler, M., Keller, T., & Cantley, L. (1988). Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature, 332(6165), 644-646.
[91]. Alzahrani, A. S., 2019, PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. In Seminars in cancer biology (Vol. 59, pp. 125-132). Academic Press.
[92]. Jayaraman, S., Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P., & Jasmine, S., 2023, Unlocking the potential of beta sitosterol: Augmenting the suppression of oral cancer cells through extrinsic and intrinsic signalling mechanisms. The Saudi Dental Journal, 35(8), 1007-1013.
[93]. Tapia, O., Riquelme, I., Leal, P., Sandoval, A., Aedo, S., Weber, H., & Roa, J. C., 2014, The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Archiv, 465, 25-33.
[94]. Porta, C., Paglino, C., & Mosca, A., 2014, Targeting PI3K/Akt/mTOR signaling in cancer. Frontiers in oncology, 4, 64.
[95]. Uddin, M. J., Shamsuzzaman, M., Horng, L., Labrique, A., Vasudevan, L., Zeller, K., Chowdhury, M., Larson, C. P., Bishai, D., & Alam, N., 2016, Use of mobile phones for improving vaccination coverage among children living in rural hard-to-reach areas and urban streets of Bangladesh. Vaccine, 34(2), 276–283. https://doi.org/10.1016/j.vaccine.2015.11.024.
[96]. O’Donnell, J. S., Massi, D., Teng, M. W., & Mandala, M., 2018, PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. In Seminars in cancer biology (Vol. 48, pp. 91-103). Academic Press.
[97]. Ramakrishnan, V., & Kumar, S., 2018, PI3K/AKT/mTOR pathway in multiple myeloma: from basic biology to clinical promise. Leukemia & lymphoma, 59(11), 2524-2534.
[98]. Gobin, B., Huin, M. B., Lamoureux, F., Ory, B., Charrier, C., Lanel, R., & Heymann, D., 2015, BYL719, a new α‐specific PI3K inhibitor: Single administration and in combination with conventional chemotherapy for the treatment of osteosarcoma. International journal of cancer, 136(4), 784-796.
[99]. Meric-Bernstam, F., Akcakanat, A., Chen, H., Do, K. A., Sangai, T., Adkins, F., & Yao, J., 2012, PIK3CA/PTEN mutations and Akt activation as markers of sensitivity to allosteric mTOR inhibitors. Clinical cancer research, 18(6), 1777-1789.
[100]. Yang, Q., & Guan, K. L., 2007, Expanding mTOR signaling. Cell research, 17(8), 666-681.
[101]. Yu, G., Wang, J., Chen, Y., Wang, X., Pan, J., Li, G., & Xie, K., 2009, Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of chinese patients with gastric cancer. Clinical Cancer Research, 15(5), 1821-1829.
[102]. Luo, Y., Xu, W., Li, G., & Cui, W., 2018, Weighing in on mTOR complex 2 signaling: the expanding role in cell metabolism. Oxidative medicine and cellular longevity, 2018(1), 7838647.
[103]. Smith, C. R., Leon, M. B., Mack, M. J., Miller, D. C., Moses, J. W., Svensson, L. G., & Pocock, S. J., 2011, Transcatheter versus surgical aortic-valve replacement in high-risk patients. New England Journal of Medicine, 364(23), 2187-2198.
[104]. Steelman, L. S., Abrams, S. L., Whelan, J., Bertrand, F. E., Ludwig, D. E., Bäsecke, J., & McCubrey, J. A., 2008, Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia, 22(4), 686-707.
[105]. Yasothkumar, D., Ramani, P., Jayaraman, S., Ramalingam, K., & Tilakaratne, W. M., 2024, Expression Profile of Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck Pathology, 18(1), 28.
[106]. Kang, X. H., Hu, W. H, Pei, S. S., 2017, Effects of Sodium Cantharidate and vitamin B6 on apoptosis and survivin expression in human osteosarcoma cells. China Medical Herald. 14, 16-19.
[107]. Hua, F., Shang, S., Hu, Z. W., 2017, Seeking new anti-cancer agents from autophagy-regulating natural products. J Asian Nat Prod Res. 19(4), 305-313.
Viewed PDF 216 5 -
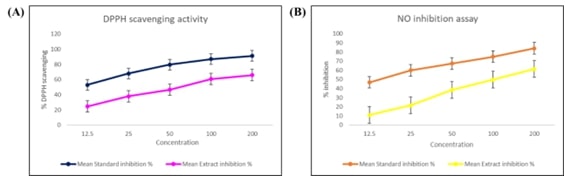 Molecular Mechanisms Underlying the Anticancer Activity of Chrysin Through p53 Tumor Suppressor in HepG2 Cell LinesAuthor: Selvaraj JayaramanDOI: 10.21522/TIJPH.2013.SE.25.01.Art024
Molecular Mechanisms Underlying the Anticancer Activity of Chrysin Through p53 Tumor Suppressor in HepG2 Cell LinesAuthor: Selvaraj JayaramanDOI: 10.21522/TIJPH.2013.SE.25.01.Art024Molecular Mechanisms Underlying the Anticancer Activity of Chrysin Through p53 Tumor Suppressor in HepG2 Cell Lines
Abstract:
Chrysin, a natural flavonoid found in passionflower, honey, and propolis, is gaining attention for its antioxidant, anti-inflammatory, and anticancer properties. This study evaluates chrysin’s anticancer efficacy against HepG2 liver cancer cells. We assessed its antioxidant potential using DPPH and nitric oxide scavenging assays, which revealed significant, concentration-dependent radical scavenging activity, emphasizing chrysin’s strong antioxidant properties. These effects are likely to reduce oxidative stress, a factor that promotes cancer cell proliferation and survival. Cytotoxicity was measured with the MTT assay, and gene expression analysis through RT-qPCR showed that chrysin upregulated pro-apoptotic genes such as Bax, Caspase 3, and Caspase 9, while downregulating the anti-apoptotic gene Bcl-2. Notably, chrysin also increased the expression of the tumor suppressor gene p53, essential for cell cycle regulation and apoptosis in response to stress and DNA damage. Molecular docking studies were performed to investigate chrysin’s interactions with key apoptotic proteins. The docking results showed strong binding affinities between chrysin and Bax, Bcl-2, Caspase 3, Caspase 9, and p53. Particularly high binding affinities with Caspase 9 and p53 suggest that chrysin may effectively trigger the intrinsic apoptotic pathway, leading to cancer cell death. The interaction with p53 is significant as it may stabilize and activate p53, enhancing the transcription of pro-apoptotic genes. These findings highlight chrysin's potential as a therapeutic agent for liver cancer, primarily through the p53-mediated apoptotic pathway. While these in vitro results are promising, further in vivo studies and clinical trials are necessary to confirm chrysin’s efficacy and safety in a clinical setting.
Molecular Mechanisms Underlying the Anticancer Activity of Chrysin Through p53 Tumor Suppressor in HepG2 Cell Lines
References:
[1]. Llovet, J. M., Kelley, R. K., Villanueva, A., Singal, A. G., Pikarsky, E., Roayaie, S., Lencioni, R., Koike, K., Zucman-Rossi, J., & Finn, R. S., 2021, Hepatocellular carcinoma. Nature Reviews. Disease Primers, 7(1), 6. https://doi.org/10.1038/s41572-020-00240-3
[2]. Khemlina, G., Ikeda, S., & Kurzrock, R., 2017, The biology of Hepatocellular carcinoma: implications for genomic and immune therapies. Molecular Cancer, 16(1), 149. https://doi.org/10.1186/s12943-017-0712-x
[3]. Hong, W., Zhang, Y., Wang, S., Zheng, D., Hsu, S., Zhou, J., Fan, J., Zeng, Z., Wang, N., Ding, Z., Yu, M., Gao, Q., & Du, S., 2024, Deciphering the immune modulation through deep transcriptomic profiling and therapeutic implications of DNA damage repair pattern in hepatocellular carcinoma. Cancer Letters, 582, 216594. https://doi.org/10.1016/j.canlet.2023.216594
[4]. Zheng, J., Chen, J., Wang, S., Yang, D., & Zhou, P., 2024, Genomic and immune landscape in hepatocellular carcinoma: Implications for personalized therapeutics. Environmental Toxicology, 39(3), 1601–1616. https://doi.org/10.1002/tox.24062
[5]. Wang, Y., & Chen, H., 2023, Protein glycosylation alterations in hepatocellular carcinoma: function and clinical implications. Oncogene, 42(24), 1970–1979. https://doi.org/10.1038/s41388-023-02702-w
[6]. Mo, Z., Liu, D., Rong, D., & Zhang, S., 2021, Hypoxic Characteristic in the Immunosuppressive Microenvironment of Hepatocellular Carcinoma. Frontiers in Immunology, 12, 611058. https://doi.org/10.3389/fimmu.2021.611058
[7]. Mani, R., & Natesan, V., 2018, Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry, 145, 187-196.
[8]. Hatano, T., Yasuhara, T., Yoshihara, R., Agata, I., Noro, T., & OKUDA, T., 1990, Effects of interaction of tannins with co-existing substances. VII.: inhibitory effects of tannins and related polyphenols on xanthine oxidase. Chemical and Pharmaceutical Bulletin, 38(5), 1224-1229.
[9]. Jayaraman, S., Natararaj, S., & Veeraraghavan, V. P., 2024, Hesperidin inhibits oral cancer cell growth via apoptosis and inflammatory signaling-mediated mechanisms: Evidence from in vitro and in silico analyses. Cureus, 16(2).
[10]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V. P., & Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition, cell cycle arrest, and metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6), 704-713.
[11]. Jayaraman, S., Veeraraghavan, V. P., Natarajan, S. R., & Jasmine, S., 2024, Exploring the therapeutic potential of curcumin in oral squamous cell carcinoma (HSC-3 cells): Molecular insights into hypoxia-mediated angiogenesis. Pathology-Research and Practice, 254, 155130.
[12]. Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Veeraraghavan, V. P., 2022, Carica papaya reduces muscle insulin resistance via IR/GLUT4 mediated signaling mechanisms in high fat diet and streptozotocin-induced type-2 diabetic rats. Antioxidants, 11(10), 2081.
[13]. Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Prasad, M., 2023, Carica papaya reduces high fat diet and streptozotocin-induced development of inflammation in adipocyte via IL-1β/IL-6/TNF-α mediated signaling mechanisms in type-2 diabetic rats. Current Issues in Molecular Biology, 45(2), 852.
[14]. Deenadayalan, A., Subramanian, V., Paramasivan, V., Veeraraghavan, V. P., Rengasamy, G., Coiambatore Sadagopan, J., & Jayaraman, S., 2021, Stevioside attenuates insulin resistance in skeletal muscle by facilitating IR/IRS-1/Akt/GLUT 4 signaling pathways: An in vivo and in silico approach. Molecules, 26(24), 7689.
[15]. Narasimhan, A., Sampath, S., Jayaraman, S., & Karundevi, B., 2013, Estradiol favors glucose oxidation in gastrocnemius muscle through modulation of insulin signaling molecules in adult female rats. Endocrine Research, 38(4), 251-262.
[16]. Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Veeraraghavan, V. P., 2022, Effect of Carica papaya on IRS-1/Akt signaling mechanisms in High-Fat-Diet–Streptozotocin-Induced type 2 diabetic experimental rats: A mechanistic approach. Nutrients, 14(19), 4181.
[17]. Perumal, S., Langeshwaran, K., Selvaraj, J., Ponnulakshmi, R., Shyamaladevi, B., & Balasubramanian, M. P., 2018, Effect of diosmin on apoptotic signaling molecules in N-nitrosodiethylamine-induced hepatocellular carcinoma in experimental rats. Molecular and Cellular Biochemistry, 449, 27-37.
[18]. Devarajan, N., Jayaraman, S., Mahendra, J., Venkatratnam, P., Rajagopal, P., Palaniappan, H., & Ganesan, S. K., 2021, Berberine—A potent chemosensitizer and chemoprotector to conventional cancer therapies. Phytotherapy Research, 35(6), 3059-3077.
[19]. Selvaraj, J., Sathish, S., Mayilvanan, C., & Balasubramanian, K., 2013, Excess aldosterone-induced changes in insulin signaling molecules and glucose oxidation in gastrocnemius muscle of adult male rat. Molecular and Cellular Biochemistry, 372, 113-126.
[20]. Venditto, V. J., & Simanek, E. E., 2010, Cancer therapies utilizing the camptothecins: A review of the in vivo literature. Molecular Pharmaceutics, 7(2), 307–349. https://doi.org/10.1021/mp900243b
Viewed PDF 205 12 -
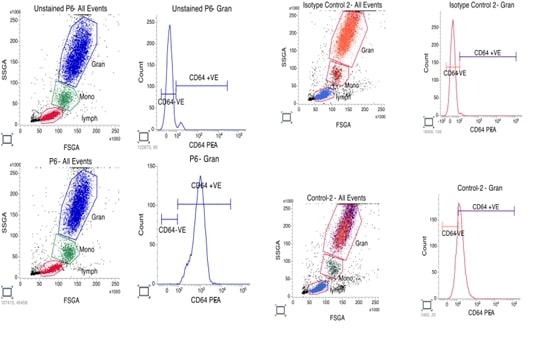 Evaluation of nCD64 in Patients with PeriodontitisAuthor: Ullas MonyDOI: 10.21522/TIJPH.2013.SE.25.01.Art025
Evaluation of nCD64 in Patients with PeriodontitisAuthor: Ullas MonyDOI: 10.21522/TIJPH.2013.SE.25.01.Art025Evaluation of nCD64 in Patients with Periodontitis
Abstract:
Periodontitis causes tissue destruction and tooth loss if untreated. Neutrophil CD64 (nCD64), a biomarker will help in the early and precise diagnosis of inflammation. Thus, the aim of this study is to evaluate the potential of neutrophil nCD64 as a diagnostic marker by looking at the expression levels of nCD64 in people with periodontitis. This study involved 12 participants comprising of 6 healthy controls and 6 of patients with periodontitis. nCD64 levels were measured on acquired blood samples using flow cytometry. Mean fluorescence intensity (MFI) of nCD64 was compared between the two groups. When compared to healthy controls, patients with periodontitis had noticeably higher nCD64 MFI levels. In a patient, the greatest nCD64 MFI level was 861, whereas in a control, the lowest level was 19. Comorbid conditions like diabetes did not always correspond with elevated nCD64 levels, suggesting that periodontitis severity was the main factor affecting nCD64 expression. The current study suggests nCD64 as a useful biomarker for identifying periodontal inflammation, which helps with the timely and precise diagnosis of periodontitis. Future studies are necessary to corroborate these results using more extensive and heterogeneous groups.
Evaluation of nCD64 in Patients with Periodontitis
References:
[1]. Mony, U., Sanju, S., Jain, P., Sugavanan, K., Sebastian, A., Theertha, M., Sidharthan, N., Varma, P. K., 2019, Detection of dysregulated host response by flow cytometry may pre-empt early diagnosis of sepsis after cardiac surgery. Blood, 134, 4863.
[2]. Ng, P. C, Li, G., Chui, K. M., Chu, W. C., Li, K., Wong, R. P, Chik, K. W, Wong, E., Fok, T. F., 2004, Neutrophil CD64 is a sensitive diagnostic marker for early-onset neonatal infection. Pediatr Res, 56(5), 796-803.
[3]. Davis, B. H., Olsen, S. H., Ahmad, E., Bigelow, N. C., 2006, Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med, 130(5), 654-61.
[4]. Naiff, P. F., Kuckelhaus, S. A., Couto, S., Oliveira, M., Santiago, L. M., Cascaes, A. C., Silva, L. F., Oliveira, L. A., Grisi, D. C., Carneiro, V. M., Guimarães, M. D. C. M., 2021, Phagocytic activity of monocytes and neutrophils in patients with periodontitis, whether or not associated to type 2 diabetes. Acta Odontol Latinoam, 34(3), 201-213.
[5]. Magán-Fernández, A., Rasheed, Al-Bakri, S. M., O'Valle, F., Benavides-Reyes, C., Abadía-Molina, F., Mesa, F., 2020, Neutrophil Extracellular Traps in Periodontitis. Cells, 9(6), 1494.
[7]. Doganyigit, Z., Eroglu, E., Akyuz, E., 2022, Inflammatory mediators of cytokines and chemokines in sepsis: From bench to bedside. Human & experimental toxicology, 41, 09603271221078871.
[8]. Liu, Q., Gao, Y., Yang, T., Zhou, Z., Lin, K., Zhang, W., Li, T., Lu, Y., Shao, L., Zhang, W., 2022, nCD64 index as a novel inflammatory indicator for the early prediction of prognosis in infectious and non-infectious inflammatory diseases: An observational study of febrile patients. Front Immunol, 13, 905060.
[9]. Wang, X., Li, Z. Y., Zeng, L., Zhang, A. Q., Pan, W., Gu, W., Jiang, J. X., 2015, Neutrophil CD64 expression as a diagnostic marker for sepsis in adult patients: a meta-analysis. Crit Care, 19(1), 245.
[10]. Hirschfeld, J., 2020, Neutrophil Subsets in Periodontal Health and Disease: A Mini Review. Front Immunol, 10, 3001.
[11]. Oppegaard, O., Skodvin, B., Halse, A. K., Langeland, N., 2013, CD64 as a potential biomarker in septic arthritis. BMC Infect Dis, 13:278.
[12]. Xing, W., Wang, Y., Liu, J., Pei, J., Yu, C., 2023, Role of interleukins in the detection of neonatal sepsis: a network meta-analysis. Frontiers in Pediatrics, 11, 1267777.
[13]. Cid, J., García-Pardo, G., Aguinaco, R., Sánchez, R., Llorente, A., 2011, Neutrophil CD64: diagnostic accuracy and prognostic value in patients presenting to the emergency department. Eur J Clin Microbiol Infect Dis, 30(7), 845-52.
[14]. Allen, E., Bakke, A. C., Purtzer, M. Z., Deodhar, A., 2002, Neutrophil CD64 expression: distinguishing acute inflammatory autoimmune disease from systemic infections. Annals of the rheumatic diseases, 61(6), 522-5.
[15]. Bakke, A. C., Allen, E., Purtzer, M. Z., Deodhar, A., 2001, Neutrophil CD64 expression distinguishing acute inflammatory autoimmune disease from systemic infections. Clinical and Applied Immunology Reviews, 1(5), 267-75.
[16]. Sanju, S., Jain, P., Vishnu Priya, V., Varma, P. K., Mony, U., 2023, Quantitation of mHLA- DR and nCD64 by Flow Cytometry to Study Dysregulated Host Response: The Use of QuantiBRITETM PE Beads and Its Stability. Appl Biochem Biotechnol, 195(9),5747-5752.
[17]. Agnes, S., S., Sanju, Jain, P., Varma, P. K., Mony, U., 2021, Non-classical monocytes and its potential in diagnosing sepsis post cardiac surgery. International Immunopharmacology, 99,108037
[18]. Hassan, U., Ghonge, T., Reddy, Jr. B., Patel, M., Rappleye, M., Taneja, I., Tanna, A., Healey, R., Manusry, N., Price, Z., Jensen, T., 2017, A point-of-care microfluidic biochip for quantification of CD64 expression from whole blood for sepsis stratification. Nature communications, 8(1),15949
[19]. Liu, Q., Gao, Y., Yang, T., Zhou, Z., Lin, K., Zhang, W., Li, T., Lu, Y., Shao, L., Zhang W., 2022, nCD64 index as a novel inflammatory indicator for the early prediction of prognosis in infectious and non-infectious inflammatory diseases: An observational study of febrile patients. Front Immunol, 13,905060.
[20]. Ghosh, P. S., Singh, H., Azim, A., Agarwal, V., Chaturvedi, S., Saran, S., Mishra, P., Gurjar, M., Baronia, A. K, Poddar, B., Singh, R. K, Mishra, R., 2018, Correlation of Neutrophil CD64 with Clinical Profile and Outcome of Sepsis Patients during Intensive Care Unit Stay. Indian J Crit Care Med, 22(8),569-574.
[21]. Renu, K., Gopalakrishnan, A.V. and Madhyastha, H., 2024. Is periodontitis triggering an inflammatory response in the liver, and does this reaction entail oxidative stress?. Odontology, pp.1-14.
[22]. Thomas, J.T., Joseph, B., Varghese, S., Kamalasanan Vijayakumari, B., Sorsa, T., Mauramo, M., Anil, S. and Waltimo, T., 2024. Salivary advanced glycated end products, their receptors, and aMMP‐8 in periodontitis patients with varying glycemic levels: A cross‐sectional study. Journal of Periodontology.
[23]. Uppin, R.B., Varghese, S.S., Baseer, M.A., AlMugeiren, O.M., Mubaraki, S. and Alsaffan, A.D., 2024. Knowledge and Awareness of Metabolic Syndrome and its Relationship with Periodontal Disease among Dental Practitioners in Riyadh City, Saudi Arabia: A Cross-Sectional Study. Journal of International Oral Health, 16(6), pp.487-497.
[24]. Yadalam, P.K., Barbosa, F.T., Natarajan, P.M. and Ardila, C.M., 2024. Graph Neural Networks-Based Prediction of Drug Gene Interactions of RTK-VEGF4 Receptor Family in Periodontal Regeneration. Journal of Clinical and Experimental Dentistry, 16(12), p.e1454.
[25]. Priyangha, P.T., Kshirsagar, J.T. and Kalaiselvan, D., 2024. Exploring Serum Ceruloplasmin Dynamics in Stage II Periodontitis: Pre-and PostPhase I Therapy Assessment. Journal of the International Clinical Dental Research Organization, 16(2), pp.135-140.
[26]. Priya, V., Keerthivasan, S. and Kaviyarasi, R., Determining the Dual Effect of Mirabegron on Anticancer Mechanism and Brown Adipose Tissue Activation-An in-silico Approach.
[27]. Juvairiya Fathima, A., Renu, K., Priya, V.V., Gayathri, R. and Kavitha, S., Determining the Role of Caffeic Acid on Lipogenic Regulators: An In-Silico Approach.
[28]. Aarthi, L., Renu, K., Priya, V.V., Gayathri, R. and Kavitha, S., Molecular Docking Analysis of Epigallocatechin 3-Gallate [EGCG] on Fatty Acids and Carnitine Transporters Family.
Hirshasri, A.G., Renu, K., Priya, V.V., Gayathri, R. and Kavitha, S., The Effect of Aspalathin on SMAD2, SMAD3, TGF-β-A Major Contributor of Inflammation–An In-silico Approach
Viewed PDF 234 16

