Table of Contents - Issue

Recent articles
-
 Idiopathic Subglottic Stenosis with Several Idiopathic Cervical and Mediastinal Lymph Nodes - Airway and Anaesthetic ManagementAuthor: Suhas MDOI: 10.21522/TIJPH.2013.SE.24.05.Art001
Idiopathic Subglottic Stenosis with Several Idiopathic Cervical and Mediastinal Lymph Nodes - Airway and Anaesthetic ManagementAuthor: Suhas MDOI: 10.21522/TIJPH.2013.SE.24.05.Art001Idiopathic Subglottic Stenosis with Several Idiopathic Cervical and Mediastinal Lymph Nodes - Airway and Anaesthetic Management
Abstract:
This case report details the anaesthetic approach for a 60-year-old female with subglottic stenosis, who had experienced bronchospasm and hypotension during previous surgeries under general anaesthesia. The patient was scheduled for open hernioplasty, and hernioplasty complex airway anatomy and history of anaesthetic complications posed significant challenges. Following a comprehensive preoperative assessment, including video laryngoscopy and CT imaging, it was decided to use a Combined Spinal Epidural (CSE) block to minimize the risks associated with general anaesthesia. The surgery was performed successfully, with the patient remaining stable throughout the procedure and during the postoperative period. This case highlights the importance of a personalized anaesthetic plan, particularly in patients with difficult airway conditions and a history of adverse reactions to general anaesthesia. Regional anaesthesia, specifically CSE, proved to be a safe and effective alternative, allowing for a successful surgical outcome without complications. This report emphasizes the need to carefully consider anaesthetic options in complex cases to enhance patient safety and optimize surgical results.
Idiopathic Subglottic Stenosis with Several Idiopathic Cervical and Mediastinal Lymph Nodes - Airway and Anaesthetic Management
References:
[1]. Erich, Stoelben., 2024, [Idiopathic Subglottic Tracheal Stenosis]. Zentralblatt Fur Chirurgie, Doi: 10.1055/a-2241-0616
[2]. Fernández MA, Bartolomé E, Villegas FR., 2009, Revisión de las estenosis traqueales trasintubación: a propósito de un caso. Medica Intensiva, 33, 301-305.
[3]. M., Direder., Maria, Laggner., Dragan, Copic., K., Klas., Daniel, Bormann., Thomas, Schweiger., Konrad, Hoetzenecker., Clemens, Aigner., Hendrik, Jan, Ankersmit., Michael, Mildner., 2024, Transcriptional profiling sheds light on the fibrotic aspects of idiopathic subglottic tracheal stenosis. bioRxiv, Doi: 10.1101/2024.02.19.580975.
[4]. Alfie, Wright., Thomas, Leahy., 2024, Atracurium-Induced Bronchospasm with Flat Capnograph at Induction of General Anaesthesia: A Case Report. Cureus, Doi: 10.7759/cureus.54251.
[5]. Brandon, Miguel, Flores, Najera., Blanca, Isela, Martínez, Montoya., 2023, Incisional Hernias: A Complete Review of Literature. Journal of Medical Science and Clinical Research. Doi: 10.47191/ijmscrs/v3-i9-20.
[6]. R., H., Fortelny., U., Dietz., 2023, [Incisional hernias: epidemiology, evidence and guidelines]. Chirurgie. Doi: 10.1007/s00104-023-01999-3.
[7]. Alexandra, D., D'Oto., Hayley, B., Baker., Ted, Mau., Lesley, F., Childs., Kathleen, Tibbetts., 2023, Characteristics of Idiopathic Subglottic Stenosis in the Elderly. Laryngoscope. Doi: 10.1002/lary.30742.
[8]. Hannan, Chaudery., Paul, Efthymiou., Stefan, R, Cozma., 2024, Cervical Necrotising Fasciitis Leading to Critical Airway Compromise: A Case Report of Successful Airway Management with Awake Fibreoptic Intubation. Cureus. Doi: 10.7759/cureus.57126.
[9]. Bedana, Maharjan., 2024, Airway management of a patient with achondroplasia using awake fiberoptic intubation: A case report. Doi: 10.1016/j.jcadva.2024.100018.
[10]. C, Pezzano., Ilana, Harwayne-Gidansky.. 2023, Use of Flow-Volume Loops on a Mechanically Ventilated Pediatric Patient as a Diagnostic Tool for Fixed Airway Obstruction. Cureus. Doi: 10.7759/cureus.39765.
[11]. Drisana, Henry., Virendra, Modi., Vaidehi, Korde., Abhijit, V., Kshirsagar., 2024, Low dose thoracic segmental spinal anaesthesia with isobaric levobupivacaine: is it an option for a patient with severe heart disease undergoing emergency cesarean section? a case study. International Journal of Scientific Research. Doi: 10.36106/ijsr/6306706.
[12]. Chang, Hoon, Choi., Hae, Jin, Lee., 2007, Anticholinergic Relieves Bronchospasm Occurring during Spinal Anesthesia in an Asthmatic Patient - A case report -. Korean Journal of Anesthesiology. Doi: 10.4097/KJAE.2007.53.2.259.
[13]. Martin, Calineata., Lukas, Jennewein., Vanessa, Neef., Armin, Niklas, Flinspach., Frank, Louwen., Kai, Zacharowski., Florian, J, Raimann., 2023, Safety and Efficiency of Low-Dose Spinal Analgesia Compared to Epidural Analgesia in Treatment of Pain during Labour: A Case Control Study. Journal of Clinical Medicine. Doi: 10.3390/jcm12185770.
[14]. Lucy, Halliday., M., Kinsella., M., Shaw., Joshua, Cheyne., Scott, M., Nelson., Rachel, J., Kearns., 2022, Comparison of ultra‐low, low and high concentration local anaesthetic for labour epidural analgesia: A systematic review and network meta‐analysis. Anaesthesia. Doi: 10.1111/anae.15756.
Viewed PDF 180 14 -
 Unveiling the Enigma: Chronic Concealed Abruption Disguised as PlacentomegalyAuthor: Deepthi. PDOI: 10.21522/TIJPH.2013.SE.24.05.Art002
Unveiling the Enigma: Chronic Concealed Abruption Disguised as PlacentomegalyAuthor: Deepthi. PDOI: 10.21522/TIJPH.2013.SE.24.05.Art002Unveiling the Enigma: Chronic Concealed Abruption Disguised as Placentomegaly
Abstract:
Here we present a case of Rh-negative Primigravida with 22 weeks gestation who presented to us with USG report of a live fetus with FGR, placentomegaly with oligohydramnios without any clinical symptoms and later diagnosed to be a case of chronic abruption oligohydramnios sequence (CAOS). Initially, all differentials of Placentomegaly like placental chorioangioma, placental mosaicism, chronic concealed abruptio placenta or placental tumours such as placental mesenchymal dysplasia were considered. Regular antenatal workup was carried out for the patient which revealed an incidental heart disease probably of rheumatic origin and the patient was managed accordingly. The patient was planned for termination of pregnancy in view of subsequent anhydramios in the midst of which the patient developed cardiac complications. The patient was stabilized and taken up for emergency hysterotomy which intraoperatively revealed retroplacental clots and altered blood as evidence of premature separation of placenta clinching the diagnosis of CAOS. Since CAOS can lead to fetal morbidity and mortality, decision-making has to be carried out regarding accelerating fetal lung maturity and neuroprotection or termination of pregnancy after carefully weighing out the fetomaternal outcomes.
Unveiling the Enigma: Chronic Concealed Abruption Disguised as Placentomegaly
References:
[1]. Weerakkody Y., 2024, Placentomegaly Radiology Reference Article Radiopaedia.org. In: Radiopaedia [Internet], Doi:10.53347/rID-13573.
[2]. Rohilla M, Siwatch S, Jain V, Nijhawan R., 2024, Placentomegaly and Placental Mesenchymal Dysplasia. BMJ Case Rep. 2012, bcr2012007777. Doi:10.1136/bcr-2012-007777.
[3]. Yonai NB, Mandel R, Shteel S, Lavie O, Goldberg Y. VP38.02: Severe Placentomegaly and Intrauterine Growth Restriction In A Persistently Hypoxemic Pregnant Woman with a Single Cardiac Ventricle. Ultrasound in Obstetrics & Gynecology. 2020;56: 222–223. Doi:10.1002/uog.22921.
[4]. Chigusa Y, Mogami H, Minamiguchi S, Kido A, Ishida A, Kurata Y, et al., 2022, Chronic abruption-oligohydramnios sequence (CAOS) revisited: possible implication of premature rupture of membranes. J Matern Fetal Neonatal Med;35: 6894–6900. Doi:10.1080/14767058.2021.1929159.
[5]. Tikkanen M, Nuutila M, Hiilesmaa V, Paavonen J, Ylikorkala O., 2006, Clinical presentation and risk factors of placental abruption. Acta Obstet Gynecol Scand. 85: 700–705. Doi:10.1080/00016340500449915.
[6]. Fleming AD., 1991, Abruptio placentae. Crit Care Clin. 7: 865–875.
[7]. Mohanty GS, Katoch T, Siwatch S, Lamba DS, Sharma RR., 2023, Chronic Abruptio Placentae with Multiple Alloantibodies: An Obstetrician’s Challenge. Cureus. 15: e47762. Doi:10.7759/cureus.47762.
[8]. Nyberg DA, Cyr DR, Mack LA, Wilson DA, Shuman WP., 1987, Sonographic spectrum of placental abruption. AJR Am J Roentgenol.148: 161–164. Doi:10.2214/ajr.148.1.161.
[9]. Harris BAJ, Gore H, Flowers CEJ., 1985, Peripheral Placental Separation: A Possible Relationship to Premature Labor. Obstetrics & Gynecology. 66: 774.
[10]. Redline RW, Wilson-Costello D., 1991, Chronic peripheral separation of placenta. The significance of diffuse chorioamnionic hemosiderosis. Am J Clin Pathol. 111: 804–810. Doi:10.1093/ajcp/111.6.804.
[11]. Jain P, Yadav R, Jaiswal N, Agarwal K., 2021, Live Pregnancy with Chronic Abruption-oligohydramnios Sequence: A Case Report. JCDR. Doi:10.7860/JCDR/2021/50455.15773.
[12]. Braun P, Kazmi K, Nogués-Meléndez P, Mas-Estellés F, Aparici-Robles F., 2007, MRI findings in spinal subdural and epidural hematomas. Eur J Radiol. 64: 119–125. Doi:10.1016/j.ejrad.2007.02.014.
Viewed PDF 172 4 -
 Effective Management of a Total Thyroidectomy Case with Positive Cuff Leak Test: A Case ReportAuthor: Kishanth kumar SDOI: 10.21522/TIJPH.2013.SE.24.05.Art003
Effective Management of a Total Thyroidectomy Case with Positive Cuff Leak Test: A Case ReportAuthor: Kishanth kumar SDOI: 10.21522/TIJPH.2013.SE.24.05.Art003Effective Management of a Total Thyroidectomy Case with Positive Cuff Leak Test: A Case Report
Abstract:
Total thyroidectomy is frequently performed to treat various thyroid conditions, such as malignancies, large goiters, and Graves' disease. One of the possible complications after this surgery is airway obstruction, which can be evaluated intraoperatively using the cuff leak test (CLT). A positive CLT suggests significant airway edema or obstruction, which can lead to postoperative respiratory difficulties. Managing a patient with a positive CLT after thyroidectomy necessitates a multidisciplinary approach, including close monitoring, prompt intervention, and appropriate postoperative care. This case shows the management of a patient who developed a positive cuff leak test following a total thyroidectomy. Key strategies involved administering corticosteroids to mitigate airway edema, ensuring close observation in an intensive care unit (ICU), and considering reintubation or tracheostomy if airway obstruction persisted. The patient was successfully managed through conservative measures, avoiding the need for invasive airway procedures. This case underscores the importance of vigilance and timely intervention in the postoperative period to secure patient safety and achieve favorable outcomes after total thyroidectomy. The discussion highlights the necessity of tailoring care plans to the severity of the airway compromise and the overall clinical condition of the patient. This case adds to the understanding of airway management in thyroidectomy patients and the utility of CLT in guiding clinical decisions.
Effective Management of a Total Thyroidectomy Case with Positive Cuff Leak Test: A Case Report
References:
[1]. Widyanti, Soewoto., Meirisa, Ardianti. 2024, Tracheomalacia following a total thyroidectomy in a patient with a large non-toxic goiter: A case report. International Journal of Surgery Case Reports, Doi: 10.1016/j.ijscr.2023.109211
[2]. Luke, Burton, Jeremy, M., Loberger., Mark, Baker., Priya, Prabhakaran., Vidit, Bhargava 2023, Pre-Extubation Ultrasound Measurement of In Situ Cuffed Endotracheal Tube Laryngeal Air Column Width Difference: Single-Center Pilot Study of Relationship with Post-Extubation Stridor in Under 5 Years Old. Pediatric Critical Care Medicine, Doi: 10.1097/pcc.0000000000003377
[3]. Aziz, Salih, Abdul-Zahra, Sajid, Hameed, Abd, Al-Helfy, Bashar, Abass, Abdulhassan 2023, Incidence and Risk Factors of Post-Thyroidectomy Stridor. Diyala Journal of Medicine, Doi: 10.26505/djm.v25i1.1023
[4]. Koji, Kanno., Naoki, Fujiwara., Takuhiro, Moromizato., Shuichi, Fujii., Yuki, Ami., A., Tokushige., Shinichiro, Ueda 2023, Pre-Extubation Cuffed Tube Leak Test and Subsequent Post-Extubation Laryngeal Edema: Prospective, Single-Center Evaluation of PICU Patients. Pediatric Critical Care Medicine, Doi: 10.1097/PCC.0000000000003282
[5]. A case of unusual presentation of post-thyroidectomy airway compromise 2023. International Journal of Scientific Research, Doi: 10.36106/ijsr/3702754
[6]. Taha, Usman, Pasha., Gul, e, Lala, Haider., Syed, Muneeb, Ali., Syed, Mujahid, Gilani., Fazal, Rabbi., Kamal, Nasir., Sana, Umar., Sayyad, Ali. 2023, Preventing Laryngeal Edema Using Single Dose Methylprednisolone in Critically Ill Patients. Journal of Bashir Institute of Health Sciences, Doi: 10.53576/bashir.004.01.0105
[7]. P., M., Diggikar., Simranbir, Bhullar., Prashant, Gopal 2023, Post-extubation Stridor in a Case of Intracranial Bleed: Assessing Airway Patency Prior to Extubation Using Cuff Leak Test. Cureus, Doi: 10.7759/cureus.33632
[8]. Dawnette, Lewis., Dev, Darshan, Khalsa., Alexandra, Cummings., James, Schneider., Sareen, Shah. 2023, Factors Associated With Post-Extubation Stridor in Infants Intubated in the Pediatric ICU. Journal of Intensive Care Medicine, Doi: 10.1177/08850666231204208
[9]. Pre-Extubation Cuffed Tube Leak Test and Subsequent Post-Extubation Laryngeal Edema: 2023, Prospective, Single-Center Evaluation of PICU Patients. Pediatric Critical Care Medicine, Doi: 10.1097/pcc.0000000000003282
[10]. Laya, Amoozadeh, Mohammad‐Taghi, Beigmohammadi., Abbas, Alipour. 2024, Gargle test for successful extubation in critically ill patients underwent head and neck surgeries: A new test. Journal of Critical Care, Doi: 10.1016/j.jcrc.2024.154755
Viewed PDF 162 7 -
 Assessing the Utility of Pregnancy-Unique Quantification of Emesis Questionnaire's Score in Managing Women with Nausea and Vomiting of PregnancyAuthor: Deepthi PDOI: 10.21522/TIJPH.2013.SE.24.05.Art004
Assessing the Utility of Pregnancy-Unique Quantification of Emesis Questionnaire's Score in Managing Women with Nausea and Vomiting of PregnancyAuthor: Deepthi PDOI: 10.21522/TIJPH.2013.SE.24.05.Art004Assessing the Utility of Pregnancy-Unique Quantification of Emesis Questionnaire's Score in Managing Women with Nausea and Vomiting of Pregnancy
Abstract:
A lower quality of life and unfavorable pregnancy outcomes are linked to the severity of nausea and vomiting during pregnancy (NVP). This study used the Pregnancy-Unique Quantification of Emesis and Nausea (PUQE) scale to compare the severity of NVP with the various demographic and maternal parameters among pregnant women. To analyse the association of severity of nausea and vomitting using a modified PUQE scale with the various demographic and maternal characteristics This was a cross-sectional study conducted on 380 pregnant women who were receiving antenatal care at a tertiary health care centre between January 2023 and March 2024. The severity of NVP was evaluated by the PUQE scale which was later compared with the various maternal characteristics. Statistical analyses were performed to determine the risk factors associated with NVP. Gestational age was significantly associated with increasing NVP. Most of the subjects in the moderate (45.2%) and severe (47.7%) PUQE group were gravida 1 while 43.9% were gravida 2 in the mild group. PUQE scale can be used to assess the severity of NVP thus aiding the healthcare professionals in providing required treatment.
Assessing the Utility of Pregnancy-Unique Quantification of Emesis Questionnaire's Score in Managing Women with Nausea and Vomiting of Pregnancy
References:
[1]. Lacasse, A., Rey, E., Ferreira, E., Morin, C., & Bérard, A., 2008, Nausea and vomiting of pregnancy: What about quality of life? BJOG: An International Journal of Obstetrics & Gynaecology, 115(12), 1484-1493.
[2]. Heitmann, K., Nordeng, H., Havnen, G. C., Solheimsnes, A., & Holst, L., 2017, The burden of nausea and vomiting during pregnancy: severe impacts on quality of life, daily life functioning and willingness to become pregnant again–results from a cross-sectional study. BMC Pregnancy and Childbirth, 17, 1-12.
[3]. Goodwin, T. M., & Ramin, S. M., 2015, Practice bulletin summary No. 153: Nausea and vomiting of pregnancy. Obstetrics and Gynecology, 126(3), 687-688.
[4]. Koren, G., Boskovic, R., Hard, M., Maltepe, C., Navioz, Y., & Einarson, A., 2002, Motherisk—PUQE (pregnancy-unique quantification of emesis and nausea) scoring system for nausea and vomiting of pregnancy. American Journal of Obstetrics and Gynecology, 186(5), S228-S231.
[5]. Fairweather, D. V., 1968, Nause and vomiting in pregnancy. American Journal of Obstetrics and Gynecology, 102(1), 135-175.
[6]. Verberg, M. F. G., Gillott, D. J., Al-Fardan, N., & Grudzinskas, J. G., 2005, Hyperemesis gravidarum, a literature review. Human Reproduction Update, 11(5), 527-539.
[7]. Vandraas, K. F., Vikanes, Å. V., Vangen, S., Magnus, P., Støer, N. C., & Grjibovski, A. M., 2013, Hyperemesis gravidarum and birth outcomes—a population‐based cohort study of 2.2 million births in the Norwegian Birth Registry. BJOG: An International Journal of Obstetrics & Gynaecology, 120(13), 1654-1660.
[8]. Birkeland, E., Stokke, G., Tangvik, R. J., Torkildsen, E. A., Boateng, J., Wollen, A. L., & Trovik, J., 2015, Norwegian PUQE (Pregnancy-Unique Quantification of Emesis and nausea) identifies patients with hyperemesis gravidarum and poor nutritional intake: a prospective cohort validation study. PloS One, 10(4), e0119962.
[9]. Choi, H. J., Bae, Y. J., Choi, J. S., Ahn, H. K., An, H. S., Yun, J. S., & Han, J. Y., 2018, Evaluation of nausea and vomiting in pregnancy using the Pregnancy-Unique Quantification of Emesis and Nausea scale in Korea. Obstetrics & Gynecology Science, 61(1), 30-37.
[10]. Kramer, J., Bowen, A., Stewart, N., & Muhajarine, N., 2013, Nausea and vomiting of pregnancy: prevalence, severity and relation to psychosocial health. MCN: The American Journal of Maternal/Child Nursing, 38(1), 21-27.
[11]. C Cicek, O. S. Y., & Demir, M. (2022). Evaluation of Nausea and Vomiting Severity in Pregnancies Conceived Through Assisted Reproduction. Gynecology Obstetrics & Reproductive Medicine, 28(1), 56-61.
[12]. Mitsuda, N., Eitoku, M., Yamasaki, K., Sakaguchi, M., Yasumitsu-Lovell, K., Maeda, N., & Suganuma, N., 2018, Nausea and vomiting during pregnancy associated with lower incidence of preterm births: the Japan Environment and Children’s Study (JECS). BMC Pregnancy and Childbirth, 18, 1-7.
[13]. Källén, B., Lundberg, G., & Åberg, A., 2003, Relationship between vitamin use, smoking, and nausea and vomiting of pregnancy. Acta Obstetricia et Gynecologica Scandinavica, 82(10), 916-920.
[14]. Bustos, M., Venkataramanan, R., & Caritis, S., 2017, Nausea and vomiting of pregnancy-What's new? Autonomic Neuroscience, 202, 62-72.s
[15]. Braunstein, G. D., & Hershman, J. M., 1976, Comparison of serum pituitary thyrotropin and chorionic gonadotropin concentrations throughout pregnancy. The Journal of Clinical Endocrinology & Metabolism, 42(6), 1123-1126.
[16]. Kaplan, P. B., Gücer, F., Sayin, N. C., Yüksel, M., Yüce, M. A., & Yardim, T., 2003, Maternal serum cytokine levels in women with hyperemesis gravidarum in the first trimester of pregnancy. Fertility and Sterility, 79(3), 498-502.
[17]. North, R. A., Whitehead, R., & Larkins, R. G., 1991, Stimulation by human chorionic gonadotropin of prostaglandin synthesis by early human placental tissue. The Journal of Clinical Endocrinology & Metabolism, 73(1), 60-70.
[18]. Davis, M. (2004). Nausea and vomiting of pregnancy: an evidence-based review. The Journal of Perinatal & Neonatal Nursing, 18(4), 312-328.
[19]. Niebyl, J. R., 2010, Nausea and vomiting in pregnancy. New England Journal of Medicine, 363(16), 1544-1550.
[20]. Roseboom, T. J., Ravelli, A. C., van der Post, J. A., & Painter, R. C., 2011, Maternal characteristics largely explain poor pregnancy outcome after hyperemesis gravidarum. European Journal of Obstetrics & Gynecology and Reproductive Biology, 156(1), 56-59.
[21]. Vikanes, Å., Skjærven, R., Grjibovski, A. M., Gunnes, N., Vangen, S., & Magnus, P. (2010). Recurrence of hyperemesis gravidarum across generations: population-based cohort study. Bmj, 340.
[22]. Trogstad, L. I., Stoltenberg, C., Magnus, P., Skjærven, R., & Irgens, L. M., 2005, Recurrence risk in hyperemesis gravidarum. BJOG: An International Journal of Obstetrics & Gynaecology, 112(12), 1641-1645.
[23]. Koren, G., Madjunkova, S., & Maltepe, C. (2014). The protective effects of nausea and vomiting of pregnancy against adverse fetal outcome—A systematic review. Reproductive Toxicology, 47, 77-80.
[24]. Chortatos, A., Haugen, M., Iversen, P. O., Vikanes, Å., Eberhard-Gran, M., Bjelland, E. K., & Veierød, M. B., 2015, Pregnancy complications and birth outcomes among women experiencing nausea only or nausea and vomiting during pregnancy in the Norwegian Mother and Child Cohort Study. BMC Pregnancy and Childbirth, 15, 1-11.
[25]. Bolin, M., Åkerud, H., Cnattingius, S., Stephansson, O., & Wikström, A. K., 2013, Hyperemesis gravidarum and risks of placental dysfunction disorders: a population‐based cohort study. BJOG: An International Journal of Obstetrics & Gynaecology, 120(5), 541-547.
[26]. Agampodi, S. B., Wickramasinghe, N. D., Horton, J., & Agampodi, T. C., 2013, Minor ailments in pregnancy are not a minor concern for pregnant women: a morbidity assessment survey in rural Sri Lanka. PloS One, 8(5), e64214.
[27]. Nurmi, M., Rautava, P., Gissler, M., Vahlberg, T., & Polo‐Kantola, P., 2020, Incidence and risk factors of hyperemesis gravidarum: a national register‐based study in Finland, 2005‐2017. Acta Obstetricia et Gynecologica Scandinavica, 99(8), 1003-1013.
[28]. Aishwarya, R., Ethirajan, S., 2022. Gestational Age at Booking for Antenatal Care in a Tertiary Healthcare Facility: A Glance. International Journal of Infertility & Fetal Medicine, 13(3), 91-95.
[29]. Rezvi, F. B., Duraisamy, R., Chaudhary, M., 2020. Oral Status of Pregnant Women-A Hospital Based Study. Int J Dentistry Oral Sci, 7(10), 881-887.
[30]. Ethirajan, S., Pritem, M. L., 2020. Study on knowledge and practice of periconceptional intake of folic acid among antenatal mothers at Saveetha Medical College Hospital, Tamil Nadu. Age, 20(25), 12-5.
Viewed PDF 173 6 -
 Twists and Turns: Navigating Clinicoradiological Presentations, Decision Making in Management and Histopathological Revelations of Adnexal TorsionAuthor: Deepthi PDOI: 10.21522/TIJPH.2013.SE.24.05.Art005
Twists and Turns: Navigating Clinicoradiological Presentations, Decision Making in Management and Histopathological Revelations of Adnexal TorsionAuthor: Deepthi PDOI: 10.21522/TIJPH.2013.SE.24.05.Art005Twists and Turns: Navigating Clinicoradiological Presentations, Decision Making in Management and Histopathological Revelations of Adnexal Torsion
Abstract:
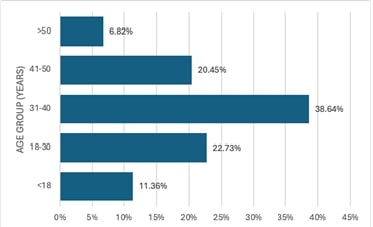
Adnexal torsion is a rare condition that can be potentially fatal if untreated. It has a varied clinical presentation and can affect any age group. Clinicians can diagnose and treat adenexal torsion more accurately if they are aware of the clinical and pathological characteristics of the patients. To analyze the clinical and pathological profile of adnexal torsion cases in Saveetha Medical College. The study was a retrospective analysis of hospital records which included all cases of adnexal torsion that underwent treatment between January 2023 to January 2024 in Saveetha Medical College. The majority (61.37%) of participants were in the reproductive age group (18-40 years). Abdominal pain was the most common symptom seen in 84.44% of patients. Majority of the women belonged to Para 2 (54.44%) and Para 1 (24.24%). Half the subjects underwent Vaginal delivery while the remaining underwent Caesarean section. A right-sided predominance was observed with most of them having one and two turns (32%). On histopathological examination, a simple serous cyst (25%) was the most predominant finding. Even though adnexal torsion is a rare clinical illness, it typically manifests as an emergency. A high degree of suspicion is necessary for the diagnosis because there are many possible clinical presentations. However, the diagnosis can only be confirmed on the operating table. The patient should be operated on as soon as possible to prevent complications and aid in conserving the ovary.
Twists and Turns: Navigating Clinicoradiological Presentations, Decision Making in Management and Histopathological Revelations of Adnexal Torsion
References:
[1]. Warner, M.A., Fleischer, A.C., Edell, S.L., Thieme, G.A., Bundy, A.L., Kurtz, A.B., et al., 1985. Uterine adnexal torsion: sonographic findings. Radiology, 154, 773–775.
[2]. Provost, R.W., 1972. Torsion of the Normal Fallopian Tube. Obstetrics & Gynecology, 39, 80.
[3]. Bider, D., Mashiach, S., Dulitzky, M., Kokia, E., Lipitz, S., Ben-Rafael, Z., 1991. Clinical, surgical and pathologic findings of adnexal torsion in pregnant and nonpregnant women. Surg Gynecol Obstet, 173, 363–366.
[4]. Vijayalakshmi, K., Reddy, G.M.M., Subbiah, V.N., Sathiya. S., Arjun, B., 2014. Clinico-pathological profile of adnexal torsion cases: a retrospective analysis from a tertiary care teaching hospital. J Clin Diagn Res, 8, OC04-07.
[5]. Verma, M., Bhuria, V., Chauhan, M., Nanda, S., Dahiya, P., Singhal, S.R., 2021. Adnexal torsion: a retrospective analysis from a tertiary care teaching hospital in northern india. Cureus, 13, e17792.
[6]. Chang, H.C., Bhatt. S., Dogra. V.S., 2008. Pearls and pitfalls in diagnosis of ovarian torsion. Radio Graphics, 28, 1355–1368.
[7]. Gupta, A., Gadipudi, A., Nayak, D., 2020. A five-year review of ovarian torsion cases: lessons learnt. J Obstet Gynaecol India, 70, 220–224.
[8]. Houry, D., Abbott, J.T., 2001. Ovarian torsion: a fifteen-year review. Ann Emerg Med, 38, 156–159.
[9]. Balci, O., Icen, M.S., Mahmoud, A.S., Capar, M., Colakoglu, M.C., 2011. Management and outcomes of adnexal torsion: a 5-year experience. Arch Gynecol Obstet, 284, 643–646.
[10]. Mashiach, R., Melamed, N., Gilad, N., Ben-Shitrit, G., Meizner. I., 2011. Sonographic diagnosis of ovarian torsion: accuracy and predictive factors. J Ultrasound Med, 30, 1205–1210.
[11]. Grunau, G.L, Harris, A., Buckley, J., Todd, N.J., 2018. Diagnosis of ovarian torsion: is it time to forget about doppler? J Obstet Gynaecol Can, 40, 871–875.
[12]. White, M., Stella, J., 2005. Ovarian torsion: 10-year perspective. Emergency Medicine Australasia, 17, 231–237.
[13]. Oltmann, S.C., Fischer, A., Barber, R., Huang, R., Hicks, B., Garcia, N., 2009. Cannot exclude torsion-a 15-year review. Journal of Pediatric Surgery, 44, 1212–1217.
[14]. Budhram, G., Elia, T., Dan, J., Schroeder, M., Safain, G., Schlech, W., et al., 2019. A Case-Control Study of Sonographic Maximum Ovarian Diameter as a Predictor of Ovarian Torsion in Emergency Department Females with Pelvic Pain. Acad Emerg Med, 26, 152–159.
[15]. Huchon, C., Fauconnier, A., 2010. Adnexal torsion: a literature review. European Journal of Obstetrics and Gynecology and Reproductive Biology, 150, 8–12.
[16]. Descargues, G., Tinlot-Mauger, F., Gravier, A., Lemoine, J.P., Marpeau, L., 2001. Adnexal torsion: a report on forty-five cases. Eur J Obstet Gynecol Reprod Biol, 98, 91–96.
[17]. Aishwarya, R., Ethirajan, S., 2022. Gestational age at booking for antenatal care in a tertiary healthcare facility: a glance. International Journal of Infertility & Fetal Medicine, 13(3), 91-95.
[18]. Omani-Samani, R., Hollins Martin, C. J., Martin, C. R., Maroufizadeh, S., Ghaheri, A., Navid, B., 2021. The birth satisfaction scale-revised Indicator (BSS-RI): a validation study in Iranian mothers. The Journal of Maternal-Fetal & Neonatal Medicine, 34(11), 1827-1831.
[19]. Sandeep, S., Shanthi, E., 2020. Study on impact of maternal age on pregnancy outcome at a tertiary care hospital. International Journal of Research in Pharmaceutical Sciences, 11(2), 235-238.
[20]. Kirubamani, N. H., 2014. Does Tubal Sterilization offer a Permanent Solution? Indian Journal of Science and Technology, 7(4), 418.
Viewed PDF 161 4 -
 Prevalence and Determinants of Gestational Diabetes Mellitus: A Cross-Sectional Study in a Suburban PopulationAuthor: Akshaya RadhakrishnanDOI: 10.21522/TIJPH.2013.SE.24.05.Art006
Prevalence and Determinants of Gestational Diabetes Mellitus: A Cross-Sectional Study in a Suburban PopulationAuthor: Akshaya RadhakrishnanDOI: 10.21522/TIJPH.2013.SE.24.05.Art006Prevalence and Determinants of Gestational Diabetes Mellitus: A Cross-Sectional Study in a Suburban Population
Abstract:
Gestational diabetes mellitus (GDM) presents significant risks to maternal and neonatal health, with varying prevalence globally. Understanding its determinants in specific populations can guide targeted interventions and improve health outcomes. This study aimed to determine the prevalence of GDM and its associated risk factors in a suburban population. A cross-sectional study was conducted among 160 pregnant women attending suburban community health centers. Data on demographic, lifestyle, and clinical variables were collected. GDM was diagnosed based on the International Association of Diabetes and Pregnancy Study Groups criteria. Statistical analyses included logistic regression to determine odds ratios (ORs) for potential risk factors. The prevalence of GDM in the study population was 15%. Significant risk factors for GDM included age ≥ 30 years (OR=2.5, 95% CI: 1.5-4.1, P=0.003), family history of diabetes (OR=3.1, 95% CI: 1.8-5.3, P=0.001), and a high sugar diet (OR=2.5, 95% CI: 1.4-4.3, P=0.001). A sedentary lifestyle and irregular meal patterns were also associated with increased GDM risk (OR=2.2 and 1.9, respectively). Overweight and obesity were strong predictors of GDM, with ORs of 2.3 and 3.5 for BMI ranges of 25-29.9 and ≥30, respectively. The study highlighted that both non-modifiable (age, family history) and modifiable (diet, lifestyle) factors significantly influence the risk of developing GDM in a suburban population. These findings suggest the necessity of targeted screening and preventive strategies that focus on modifiable lifestyle adjustments in at-risk groups.
Prevalence and Determinants of Gestational Diabetes Mellitus: A Cross-Sectional Study in a Suburban Population
References:
[1]. Dewi, R.S., Isfandiari, M.A., Martini, S., & Yi-Li, C., 2023, Prevalence and risk factors of gestational diabetes mellitus in Asia: A review. Journal of Public Health in Africa, 14(2), 7. https://doi.org/10.4081/jphia.2023.1460
[2]. Beyene, F.Y., Kassa, B.G., Mihretie, G.N., & Ayele, A.D., 2023, Gestational diabetes mellitus and its associated factors in Ethiopia: A systematic review and meta-analysis. European Journal of Medical Research, 28(1), 125. https://doi.org/10.1186/s40001-023-01433-2
[3]. Gajera, D., Trivedi, V., Thaker, P., Rathod, M., & Dharamsi, A., 2023, Detailed review on gestational diabetes mellitus with emphasis on pathophysiology, epidemiology, related risk factors, and its subsequent conversion to type 2 diabetes mellitus. Hormone and Metabolic Research, 55(05), 295-303. https://doi.org/10.1055/a-2118-2025
[4]. Siddique, E., Saddique, H., & Batool, S., 2023, Prevalence of gestational diabetes and associated maternal factors: Prevalence of gestational diabetes. Pakistan Journal of Health Sciences, 4(2), 253-258. https://doi.org/10.52229/pjhs.v4i02.582
[5]. Monod, C., Kotzaeridi, G., Linder, T., Eppel, D., Rosicky, I., Filippi, V., Tura, A., Hösli, I., & Göbl, C.S., 2023, Prevalence of gestational diabetes mellitus in women with a family history of type 2 diabetes in first- and second-degree relatives. Acta Diabetologica, 60(3), 345-351. https://doi.org/10.1007/s00592-023-02056-3
[6]. Bruno, B., & Terna, G., 2023, Rising prevalence of gestational diabetes mellitus and its associated risk factors in Makurdi, North-Central Region of Nigeria. African Health Sciences, 23(4), 348-355. https://doi.org/10.4314/ahs.v23i4.40
[7]. Orós, M., Perejón, D., Serna, M. C., Siscart, J., Leon, J., Ortega, M., & Salinas-Roca, B., 2023, Prevalence and risk factors of gestational diabetes in the health region of Lleida: A retrospective observational cohort study. Journal of Endocrinological Investigation, 46(12), 2639-2646. https://doi.org/10.1007/s40618-023-01927-4
[8]. Scheuer, C. M., Andersen, M. H., Mathiesen, E. R., Ringholm, L., Müller, C. L., Truong, J.M., Lie-Olesen, M.M., Overgaard, M., McIntyre, H.D., Jensen, D.M., & Damm, P., 2023, Regional divergence and time trends in the prevalence of gestational diabetes mellitus: A national Danish cohort study. Acta Diabetologica, 60(3), 379-386. https://doi.org/10.1007/s00592-023-02041-w
[9]. Roustaei, Z., Anttonen, S., Räisänen, S., Gissler, M., & Heinonen, S., 2023, Socioeconomic status, maternal risk factors, and gestational diabetes mellitus across reproductive years: A Finnish register-based study. BMJ Open Diabetes Research and Care, 11(4), e003278. https://doi.org/10.1136/bmjdrc-2023-003278
[10]. Liu, Q., Chen, X., Wei, S., & Wang, F., 2023, Prevalence of gestational diabetes mellitus and associated factors in Shenzhen, China: A retrospective analysis of 70,427 pregnant women. International Journal of Diabetes in Developing Countries, 43(4), 517-522. https://doi.org/10.1007/s13410-023-01088-7
[11]. Deitch, J., Yates, C. J., Hamblin, P. S., Kevat, D., Shahid, I., Teale, G., Lee, I., 2023, Prevalence of gestational diabetes mellitus, maternal obesity and associated perinatal outcomes over 10 years in an Australian tertiary maternity provider. Diabetes Research and Clinical Practice, 203, 110793. https://doi.org/10.1016/j.diabres.2023.110793
[12]. Francis, E. C., Powe, C. E., Lowe Jr, W. L., White, S. L., Scholtens, D.M., Yang, J., Zhu, Y., Zhang, C., Hivert, M.F., Kwak, S.H., & Sweeting, A., 2023, Refining the diagnosis of gestational diabetes mellitus: A systematic review and meta-analysis. Communications Medicine, 3(1), 185. https://doi.org/10.1038/s43856-023-00240-1
[13]. Sadeghi, S., Khatibi, S. R., Mahdizadeh, M., Peyman, N., & Dorniani, S.Z., 2023, Prevalence of gestational diabetes in Iran: A systematic review and meta-analysis. Medical Journal of the Islamic Republic of Iran, 37. https://doi.org/10.47176/mjiri.37.83
Viewed PDF 178 2 -
 Risk Behaviour and Psychosocial Stressors of Clients Attending Integrated Counseling and Testing Centre (ICTC) of a Rural Community Health Centre, Tamil NaduAuthor: Lakshmi ThangaveluDOI: 10.21522/TIJPH.2013.SE.24.05.Art007
Risk Behaviour and Psychosocial Stressors of Clients Attending Integrated Counseling and Testing Centre (ICTC) of a Rural Community Health Centre, Tamil NaduAuthor: Lakshmi ThangaveluDOI: 10.21522/TIJPH.2013.SE.24.05.Art007Risk Behaviour and Psychosocial Stressors of Clients Attending Integrated Counseling and Testing Centre (ICTC) of a Rural Community Health Centre, Tamil Nadu
Abstract:
India has the third largest HIV epidemic in the world, with an adult (15-49 years) HIV prevalence of 0.22%. Lack of knowledge and social taboos related to sex issues are major contributing factors to the spread of HIV/AIDS. The present study is aimed to assess risk behaviour and psychosocial stressors of voluntary attendees of ICTC, towards HIV/AIDS in a rural community health centre in Tamil Nadu. A total of 184 attendees at ICTC, either attending voluntarily or referred from different primary health centres were included. A pre-designed structured questionnaire was self -self-administered to each client to evaluate risky behaviour and psychosocial stressors about HIV/AIDS. Heterosexual risky behaviour 133(72.3%) was the most common risky sexual behaviour among the study subjects. Among primarily anticipated psychosocial stressors financial burden 60 (32.6%) was the most common concern among the attendees followed by the fear of Serious illness 58 (31.5%). The present study identifies the various risk behaviours which might be responsible for the occurrence of HIV infection among the study participants and the present study also brought out the various psychosocial stressors faced by the clients attending ICTC, so that counseling techniques to tackle such stressors can be incorporated in the training module of the counsellors to impart better counseling services. Health education can be given to the clients to reduce the risk behaviour and also to allay the misbeliefs regarding HIV/AIDS.
Risk Behaviour and Psychosocial Stressors of Clients Attending Integrated Counseling and Testing Centre (ICTC) of a Rural Community Health Centre, Tamil Nadu
References:
[1]. Government of India; National AIDS Control Organization (NACO) National HIV Counseling And Testing Services (Hcts) Guidelines; December 2016; available from URL: http://naco.gov.in/integrated-Counseling-and-testing-centre
[2]. Jennifer JKish-Gephart. Social class & risk preferences and behaviour. Current Opinion in Psychology December 2017; volume:18; 89-92; available from URL: https://www.sciencedirect.com/science/article/pii/S2352250X17300970
[3]. Sam M. S., "Psychosocial Stressor," in Psychology Dictionary.org, April 28, 2013; Available from URL; https://psychologydictionary.org/psychosocial-stressor
[4]. UNAIDS Global AIDS Update—Confronting inequalities—Lessons for pandemic responses from 40 years of AIDS [cited on JULY 14, 2021] pg.no. 14 Available from URL https://www.unaids.org/sites/default/files/media_asset/2021-global-aids-update_en.pdf
[5]. National AIDS Control Organization. Sankalak: Status of National AIDS Response (Second edition, 2020) (p. 17). Available from URL:http://naco.gov.in/sites/default/files/Sankalak_Report.pdf
[6]. National AIDS Control Organisation (NACO). Operational Guidelines for Integrated Counseling and Testing Services. New Delhi: NACO; 2007, Available from URL: http://naco.gov.in/sites/default/files/Operational%20Guidelines%20for%20Integrated%20Counseling%20and%20Testing%20Centres.pdf
[7]. National AIDS Control Organisation & ICMR-National Institute of Medical Statistics, 2021, India HIV Estimates, 2020, Technical Brief. New Delhi: NACO, Ministry of Health and Family Welfare, Government of India. 22 URL:http://naco.gov.in/sites/default/files/India%20HIV%20Estimates%202020__Web_Version.pdf
[8]. The Sustainable Development Goals and the UNAIDS Strategy, 2017. Available from URL: http://www.icad-cisd.com/pdf/Publications/SDGs-and-UNAIDS-Strategy-.pdf
[10]. Chauhan, T., Bhardwaj, A. K., Parashar, A., Kanga, A. K., 2013, A study of Knowledge, Attitude, Behaviour and practice (KABP) among the attendees of the Integrated Counseling and Testing Centre of Tertiary Care Hospital of Northern Hilly State of India. Al Ameen J Med Sci.; 6(3): 265-71.
[11]. Nayak, R. K., Kulkarni, R. D., Ajantha, G. S., 2019, Trend of clients attending an integrated councelling and testing centre of a tertiary care hospital in North Karnataka: A record based study. Int J Community Med Public Health, 6(7), 2977-2981.
[12]. Kumar, P. P., John, S., Cherian, A. V., Pandian, R. D., Anand, N., Rao, T. S., 2024, Awareness and knowledge of integrated Counseling and testing centres (ICTC) counsellors about depression among people with human immunodeficiency virus (HIV): A descriptive study from Karnataka. Industrial Psychiatry Journal, 33(1), 48-53.
[13]. Onubi, J., Adeola, O. A., Eseigbe, P., Elisha, A., Sheyin, G. O., Chima, A. A. G., 2024, Sociodemographic Factors Associated with Depression among People Living with Human Immunodeficiency Virus on Antiretroviral Therapy at a University Teaching Hospital in a Nigerian Cosmopolitan City. Exploratory Research and Hypothesis in Medicine, (000), 0-0.
[14]. Amoko, A., Ayinmode, B. A., Odeigah, L. O., Alabi, K. M., Ajiboye, P. O., Adunmo, E. O. 2016. Prevalence of depression and socio-demographic characteristics of HIV-infected patients seen by family physicians at University of Ilorin Teaching Hospital, Ilorin, Nigeria. Nigerian Journal of Family Practice, 7(1), 7-15.
[15]. Hiremath, S. B., & Desai, M., 2017, A study on prevalence and correlates of depression among women living with human immunodeficiency virus/acquired immune deficiency syndrome in North Karnataka. Industrial Psychiatry Journal, 26(2), 188-193.
[16]. Yee, T. M., Gee, M. L. H., Guan, N. C., Teong, J. T., Kamarulzaman, A., 2009, Identifying depression among the human immunodeficiency virus (HIV) patients in University Malaya Medical Centre, Kuala Lumpur, Malaysia. ASEAN Journal of Psychiatry, 10(2), 1-13.
[17]. Dhir, D., Kaur, R., Chaudhary, V., Devi, V., Kumari, S., Kumar, R., Pal, B., 2024, Prevalence of depression and associated factors among HIV/AIDS patients in India: a systematic review and meta-analysis. Journal of HIV/AIDS & Social Services, 1-19.
[18]. Sivamalar, S., Gomathi, S., Boobalan, J., Balakrishnan, P., Pradeep, A., Devaraj, C. A. Saravanan, S., 2024, Delayed identification of treatment failure causes high levels of acquired drug resistance and less future drug options among HIV-1-infected South Indians. Indian Journal of Medical Microbiology, 47, 100520.
[19]. Sivamalar, S., Gomathi, S., Boobalan, J., Balakrishnan, P., Pradeep, A., Devaraj, C. A. Saravanan, S., 2024, Delayed identification of treatment failure causes high levels of acquired drug resistance and less future drug options among HIV-1-infected South Indians. Indian Journal of Medical Microbiology, 47, 100520.
[20]. Sambandan, E., Thenmozhi, K., Santosh, G., Wang, C. C., Tsai, P. C., Gurrani, S Ponnusamy, V. K., 2024, Identification and simultaneous quantification of potential genotoxic impurities in first-line HIV drug dolutegravir sodium using fast ultrasonication-assisted extraction method coupled with GC–MS and in-silico toxicity assessment. Journal of Chromatography B, 1245, 124275.
Viewed PDF 152 3 -
 MicroRNAs and Apoptosis Signaling Pathways in Breast Cancer: From Molecular Insights to Clinical ApplicationsAuthor: Prakash BaluDOI: 10.21522/TIJPH.2013.SE.24.05.Art008
MicroRNAs and Apoptosis Signaling Pathways in Breast Cancer: From Molecular Insights to Clinical ApplicationsAuthor: Prakash BaluDOI: 10.21522/TIJPH.2013.SE.24.05.Art008MicroRNAs and Apoptosis Signaling Pathways in Breast Cancer: From Molecular Insights to Clinical Applications
Abstract:
Breast cancer represents a global health concern, necessitating a deeper understanding of the intricate mechanisms underlying its pathogenesis and therapy resistance. This comprehensive review article explores the pivotal roles of microRNAs (miRNAs) and apoptosis signaling pathways in breast cancer biology. MiRNAs, as essential post-transcriptional regulators, modulate gene expression and play a central role in apoptosis regulation. We examine their involvement in breast cancer oncogenesis, metastasis, and therapy resistance, highlighting pro-apoptotic and anti-apoptotic miRNAs. Additionally, we delve into the core components of the apoptosis pathway, including initiator and executioner caspases and Bcl-2 family members, emphasizing their relevance in breast cancer. Further, we explore the crosstalk between miRNAs and major signaling pathways (PI3K/AKT, NF-κB, and p53) and discuss their clinical implications, including diagnostics, prognostics, and therapeutic interventions. While offering promising avenues for breast cancer management, this review also identifies research gaps and challenges in translating miRNA and pathway-based knowledge into clinical practice.
MicroRNAs and Apoptosis Signaling Pathways in Breast Cancer: From Molecular Insights to Clinical Applications
References:
[1]. Savarese, G., Becher, P. M., Lund, L. H., Seferovic, P., Rosano, G.M., Coats, A. J., 2022. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovascular Research, 118(17), pp.3272-3287.
[2]. Anwar, S., Yokota, T., 2023. Navigating the complex landscape of fibrodysplasia ossificans progressiva: From current paradigms to therapeutic frontiers. Genes, 14(12), 2162.
[3]. Mirzayans, R., Murray, D., 2022. What are the reasons for continuing failures in cancer therapy? Are misleading/inappropriate preclinical assays to be blamed? Might some modern therapies cause more harm than benefit? International Journal of Molecular Sciences, 23(21), 13217.
[4]. Griffin, K. H., Fok, S. W., Kent Leach, J., 2022. Strategies to capitalize on cell spheroid therapeutic potential for tissue repair and disease modeling. NPJ Regenerative Medicine, 7(1), 70.
[5]. Rahman, M. M., Islam, M. R., Shohag, S., Ahasan, M. T., Sarkar, N., Khan, H., Rauf, A., 2022. Microbiome in cancer: Role in carcinogenesis and impact in therapeutic strategies. Biomedicine & Pharmacotherapy, 149, 112898.
[6]. Asghariazar, V., Kadkhodayi, M., Sarailoo, M., Jolfayi, A. G., Baradaran, B., 2023. MicroRNA-143 as a potential tumor suppressor in cancer: An insight into molecular targets and signaling pathways. Pathology-Research and Practice, 154792.
[7]. Pekarek, L., Torres-Carranza, D., Fraile-Martinez, O., García-Montero, C., Pekarek, T., Saez, M. A., Ortega, M. A., 2023. An overview of the role of MicroRNAs on carcinogenesis: a focus on cell cycle, angiogenesis and metastasis. International Journal of Molecular Sciences, 24(8), 7268.
[8]. Cumming, T., Levayer, R., 2024. Toward a predictive understanding of epithelial cell death. In Seminars in Cell & Developmental Biology 156, 44-57. Academic Press.
[9]. Al-Harbi, L. N., Al-Shammari, G. M., Subash-Babu, P., Mohammed, M. A., Alkreadees, R. A., Yagoub, A. E. A., 2022. Cinchona officinalis phytochemicals-loaded iron oxide nanoparticles induce cytotoxicity and stimulate apoptosis in MCF-7 human breast cancer cells. Nanomaterials, 12(19), 3393.
[10]. Sarosiek, K. A., Wood, K. C., 2023. Endogenous and imposed determinants of apoptotic vulnerabilities in cancer. Trends in Cancer, 9(2), 96-110.
[11]. Winder, M. L., Campbell, K. J., 2022. MCL-1 is a clinically targetable vulnerability in breast cancer. Cell Cycle, 21(14), 1439-1455.
[12]. Martelli, A., Omrani, M., Zarghooni, M., Citi, V., Brogi, S., Calderone, V., Ghavami, S., 2022. New visions on natural products and cancer therapy: autophagy and related regulatory pathways. Cancers, 14(23), 5839.
[13]. Hashemi, M., Mirdamadi, M. S. A., Talebi, Y., Khaniabad, N., Banaei, G., Daneii, P., Khan, H., 2023. Pre-clinical and clinical importance of miR-21 in human cancers: Tumorigenesis, therapy response, delivery approaches and targeting agents. Pharmacological Research, 187, 106568.
[14]. Huang, Y., Hong, W., Wei, X., 2022. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. Journal of Hematology & Oncology, 15(1), 129.
[15]. Doghish, A. S., Abulsoud, A. I., Elshaer, S. S., Abdelmaksoud, N. M., Zaki, M. B., El-Mahdy, H. A., Elsakka, E. G., 2023. miRNAs as Cornerstones in Chronic Lymphocytic Leukemia Pathogenesis and Therapeutic Resistance–An emphasis on the interaction of signaling pathways. Pathology-Research and Practice, 243, 154363.
[16]. Sweef, O., Zaabout, E., Bakheet, A., Halawa, M., Gad, I., Akela, M., Furuta, S., 2023. Unraveling therapeutic opportunities and the diagnostic potential of microRNAs for human lung cancer. Pharmaceutics, 15(8), 2061.
[17]. Li, C., Weng, S., He, J., 2019. WSSV–host interaction: Host response and immune evasion. Fish & Shellfish Immunology, 84, 558-571.
[18]. Tavares, N. T., Henrique, R., Bagrodia, A., Jerónimo, C., Lobo, J., 2023. A stroll through the present and future of testicular germ cell tumour biomarkers. Expert Review of Molecular Diagnostics, 23(5), 405-418.
[19]. Sastri, K. T., Gupta, N. V., Kannan, A., Balamuralidhara, V., Ramkishan, A., 2022. Potential nanocarrier-mediated miRNA-based therapy approaches for multiple sclerosis. Drug Discovery Today, 27(11), 103357.
[20]. Rossi, M., Steklov, M., Huberty, F., Nguyen, T., Marijsse, J., Jacques-Hespel, C., Breman, E., 2023. Efficient shRNA-based knockdown of multiple target genes for cell therapy using a chimeric miRNA cluster platform. Molecular Therapy-Nucleic Acids, 34.
[21]. Swain, S. S., Pati, S., Hussain, T., 2022. Quinoline heterocyclic containing plant and marine candidates against drug-resistant Mycobacterium tuberculosis: A systematic drug-ability investigation. European Journal of Medicinal Chemistry, 232, 114173.
[22]. Swain, S. S., Pati, S., Hussain, T., 2022. Quinoline heterocyclic containing plant and marine candidates against drug-resistant Mycobacterium tuberculosis: A systematic drug-ability investigation. European Journal of Medicinal Chemistry, 232, 114173.
[23]. Prasanth, G., Anbumaran, P. M., Swetha, S., Krishnarajasekhar, O. R., Gangadharan, V., 2023. Medical thoracoscopy–Diagnostics of pleural effusion with indefinite etiology. Biomedicine, 43(01), 507-513.
[24]. Balusamy, S. R., Perumalsamy, H., Veerappan, K., Huq, M. A., Rajeshkumar, S., Lakshmi, T., Kim, Y. J., 2020. Citral induced apoptosis through modulation of key genes involved in fatty acid biosynthesis in human prostate cancer cells: In silico and in vitro study. BioMed research international, 2020(1), 6040727.
[25]. Tansushree, B., Magendran, J. A., 2020. Study on awareness of breast cancer among nursing students. Indian J Forensic Med Toxicol, 14, 152-7.
Viewed PDF 223 9 -
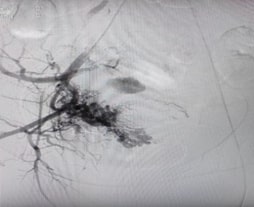 A Case of Retained Products of Conception Adherent to the Caesarean Scar Site with AV Malformation Managed with Uterine Artery EmbolisationAuthor: Deepthi PDOI: 10.21522/TIJPH.2013.SE.24.05.Art009
A Case of Retained Products of Conception Adherent to the Caesarean Scar Site with AV Malformation Managed with Uterine Artery EmbolisationAuthor: Deepthi PDOI: 10.21522/TIJPH.2013.SE.24.05.Art009A Case of Retained Products of Conception Adherent to the Caesarean Scar Site with AV Malformation Managed with Uterine Artery Embolisation
Abstract:
Retained products of conception (RPOC) and uterine arteriovenous malformations (AVM) are uncommon but serious causes of abnormal uterine bleeding (AUB). This case involves a 29-year-old woman, Para 2 Live 2, with a history of two caesarean sections and two previous abortions, who experienced heavy menstrual bleeding following medical termination of pregnancy (MTP) and tubectomy. Initially managed conservatively with methotrexate, she presented again a month later with recurrent AUB. Ultrasound and Doppler imaging revealed a thickened endometrium with significant vascularity, indicating RPOC and uterine AVM. Her β-HCG levels were elevated (47.13) but trended downward upon repeat testing. Based on interventional radiology advice, she underwent bilateral uterine artery embolization (UAE) with gel foam. Following the procedure, the patient's symptoms improved significantly, with a noticeable reduction in both the frequency and intensity of her bleeding episodes. Her β-HCG levels continued to decline, and no further episodes of heavy bleeding were noted during the one-month follow-up. This case highlights the diagnostic challenges associated with RPOC and uterine AVM, particularly after abortion. Doppler ultrasound plays a key role in detecting vascular abnormalities. Uterine artery embolization is an effective and safe first-line treatment, reducing hemorrhage risk and the need for hysterectomy. Early identification and intervention are critical to prevent life-threatening complications and ensure a favorable outcome.
A Case of Retained Products of Conception Adherent to the Caesarean Scar Site with AV Malformation Managed with Uterine Artery Embolisation
References:
[1]. Mishra, V., Chhetry. M., Pathak, K., Choudhary, S., 2024. Uterine Artery Embolization for Acquired Arteriovenous Malformation in Young Patients with Abnormal Uterine Bleeding: From Diagnosis to Management Asking the Right Questions! Insights from a Case Report. Journal of South Asian Federation of Obstetrics and Gynaecology, 16, 322–324. Doi:10.5005/jp-journals-10006-2416.
[2]. Yoon, D. J., Jones, M., Taani, J. A., Buhimschi, C., Dowell, J. D., 2016. A Systematic Review of Acquired Uterine Arteriovenous Malformations: Pathophysiology, Diagnosis, and Transcatheter Treatment. AJP Reports, 6, e6. Doi:10.1055/s-0035-1563721.
[3]. Timmerman, D., Wauters, J., Van Calenbergh, S., Van Schoubroeck, D., Maleux, G., Van Den Bosch, T., et al., 2003. Color Doppler imaging is a valuable tool for the diagnosis and management of uterine vascular malformations. Ultrasound in Obstetrics & Gynecology, 21, 570–577. Doi:10.1002/uog.159.
[4]. Sambashivaiah, J., Velayudam, L., Tigga, M., Manoli, N., 2020. Uterine arteriovenous malformation as a cause of secondary postpartum hemorrhage: A case report. Journal of South Asian Federation of Obstetrics and Gynaecology, 12(3), 189.
[5]. Sellmyer, M., Desser, T., Maturen, K., Jeffrey, R., Kamaya, A., 2013. Physiologic, histologic, and imaging features of retained products of conception. Radiographics : A review publication of the Radiological Society of North America Inc, 33, 781–796. Doi:10.1148/rg.333125177.
[6]. Van Den Bosch, T., Van Schoubroeck, D., Lu, C., De Brabanter, J., Van Huffel, S., Timmerman D., 2020. Color Doppler and gray-scale ultrasound evaluation of the postpartum uterus. Ultrasound in Obstetrics Gynecology, 20, 586–591. Doi:10.1046/j.1469-0705.2002.00851.x.
[7]. Annaiah, T., Sreenivasan, S., 2015. Uterine arteriovenous malformations: clinical implications. The Obstetrician & Gynaecologist, 17, 243–250. Doi:10.1111/tog.12218.
[8]. Kitahara, T., Sato, Y., Kakui, K., Tatsumi, K., Fujiwara, H., Konishi, I. 2011. Management of retained products of conception with marked vascularity. Journal of Obstetrics and Gynaecology Research, 37(5), 458-464.
[9]. Van den Bosch, T., Van Schoubroeck, D., Timmerman, D., 2015. Maximum Peak Systolic Velocity and Management of Highly Vascularized Retained Products of Conception. Journal of Ultrasound in Medicine Official Journal of the American Institute of Ultrasound in Medicine, 34. 1577–1582. Doi:10.7863/ultra.15.14.10050.
[10]. Kamaya, A., Petrovitch, I., Chen, B., Frederick, C. E., Jeffrey, R. B., 2009. Retained products of conception: spectrum of color Doppler findings. J Ultrasound Med. 28: 1031–1041. Doi:10.7863/jum.2009.28.8.1031.
[11]. Jain, K., Fogata, M., 2007. Retained products of conception mimicking a large endometrial AVM: Complete resolution following spontaneous abortion. Journal of Clinical Ultrasound Jcu, 35. Doi:10.1002/jcu.20250.
[12]. Bazeries, P., Paisant-Thouveny, F., Yahya, S., Bouvier, A., Nedelcu, C., Boussion, F., Aubé, C. 2017. Uterine artery embolization for retained products of conception with marked vascularity: a safe and efficient first-line treatment. Cardiovascular and Interventional Radiology, 40. Doi:10.1007/s00270-016-1543-7.
[13]. Kido, A., Togashi, K., Koyama, T., Ito H, Tatsumi K, Fujii S, et al., 2003. Retained products of conception masquerading as acquired arteriovenous malformation. J Comput Assist Tomogr, 27, 88–92. Doi:10.1097/00004728-200301000-00016.
[14]. Cohen, S. B., Kalter-Ferber, A., Weisz, B. S., Zalel, Y., Seidman, D. S., Mashiach, S., Goldenberg, M., 2001. Hysteroscopy may be the method of choice for management of residual trophoblastic tissue. The Journal of the American Association of Gynecologic Laparoscopists, 8, Doi:10.1016/s1074-3804(05)60577-4.
[15]. Grivell, R. M., Reid, K. M., Mellor, A., 2005. Uterine arteriovenous malformations: a review of the current literature. Obstet Gynecol Surv, 60, 761–767. Doi: 10.1097/01.ogx.0000183684.67656.ba.
[16]. Hickey, M., Fraser, I. S., 2000. Clinical implications of disturbances of uterine vascular morphology and function. Baillieres Best Pract Res Clin Obstet Gynaecol, 14, 937–951. Doi:10.1053/beog.2000.0136.
[17]. Sugiyama, T., Honda, S., Kataoka, A., Komai, K., Izumi, S., Yakushiji. M., 1996. Diagnosis of uterine arteriovenous malformation by color and pulsed Doppler ultrasonography. Ultrasound in Obstetrics & Gynecology, 8. 359–360. Doi:10.1046/j.1469-0705.1996.08050355-3.x.
[18]. Hoffman, M. K., Meilstrup, J. W., Shackelford. D. P., Kaminski, P. F., 1997. Arteriovenous malformations of the uterus: An uncommon cause of vaginal bleeding. Obstetrical & Gynecological Survey, 52: 736.
[19]. Qian, Z. D., Weng, Y., Du, Y. J., Wang, C. F., & Huang, L. L., 2017. Management of persistent caesarean scar pregnancy after curettage treatment failure. BMC Pregnancy and Childbirth, 17: 208. Doi:10.1186/s12884-017-1395-4.
[20]. Vilos, A. G., Vilos, G. A., Hollett-Caines, J., Rajakumar, C., Garvin, G., Kozak, R., 2015. Uterine artery embolization for uterine arteriovenous malformation in five women desiring fertility: pregnancy outcomes. Human Reproduction, 30, 1599–1605. Doi:10.1093/humrep/dev097.
[21]. Joffre, F., Tubiana, J, M., Pelage, J.P., 2004. FEMIC: Uterine fibroid embolization using tris-acryl microspheres. A french multicenter study. cardiovascular and interventional radiology, 27, 600–6. Doi:10.1007/s00270-004-0078-5.
[22]. Peitsidis, P., Manolakos, E., Tsekoura, V., Kreienberg, R., Schwentner, L., 2011. Uterine arteriovenous malformations induced after diagnostic curettage: a systematic review. Archives of Gynecology and Obstetrics, 284, 1137-1151.
[23]. Maleux, G., Timmerman, D., Heye, S., Wilms, G., 2006, Acquired uterine vascular malformations: Radiological and clinical outcome after transcatheter embolotherapy. European Radiology, 16, 299–306. Doi:10.1007/s00330-005-2799-5.
[24]. Salomon, L. J., de Tayrac, R., Castaigne‐Meary, V., Audibert, F., Musset, D., Ciorascu, R., et al., 2003. Fertility and pregnancy outcome following pelvic arterial embolization for severe post‐partum haemorrhage. A cohort study. Human Reproduction, 18, 849–852. Doi:10.1093/humrep/deg168.
[25]. Nijamudeen, S. S., 2020. A Study of Awareness on Artificial Insemination among Medical College Students. Indian Journal of Forensic Medicine & Toxicology, 14(3).
[26]. Ramesh, A., Chander, R. V., Srinivasan, C., Vengadassalapathy, S. 2020. Prevalence of angiogenesis, proliferation, and apoptosis markers of cervical cancer and their correlation with clinicopathological parameters. Journal of Oncology, 2020(1), 8541415.
[27]. Omani-Samani, R., Hollins Martin, C. J., Martin, C. R., Maroufizadeh, S., Ghaheri, A., Navid, B., 2021. The birth satisfaction scale-revised Indicator (BSS-RI): A validation study in Iranian mothers. The Journal of Maternal-Fetal & Neonatal Medicine, 34(11), 1827-1831.
Viewed PDF 210 4 -
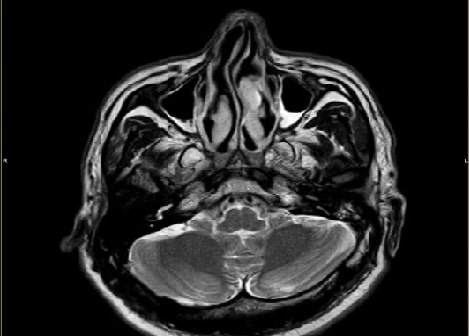 A Case of CSF Rhinorrhoea Presenting as Recurrent MeningitisAuthor: Sriharsha TDOI: 10.21522/TIJPH.2013.SE.24.05.Art010
A Case of CSF Rhinorrhoea Presenting as Recurrent MeningitisAuthor: Sriharsha TDOI: 10.21522/TIJPH.2013.SE.24.05.Art010A Case of CSF Rhinorrhoea Presenting as Recurrent Meningitis
Abstract:
This case report describes a 40-year-old male patient who experienced recurrent meningitis with a history of CSF rhinorrhoea. The first episode of meningitis caused by Streptococcus pneumoniae was successfully treated with antibiotics. MRI cisternography confirmed the presence of CSF rhinorrhoea, demonstrating CSF leakage from the subarachnoid space through defects in the left fovea ethmoidalis and lateral lamella into the left frontal sinus and left anterior ethmoidal sinus. The patient was referred to a neurosurgeon for repair of the CSF leak, which was successfully performed via bifrontal craniotomy and anterior cranial fossa (ACF) base repair through an eyebrow approach. The patient recovered fully following antibiotic and steroid regimens, and subsequent follow-up after one year revealed no recurrence of the meningitis symptoms. This case highlights the importance of considering CSF rhinorrhoea as a potential cause of recurrent meningitis, and the need for prompt diagnosis and treatment to prevent complications.
A Case of CSF Rhinorrhoea Presenting as Recurrent Meningitis
References:
[1]. Tebruegge, M., Curtis, N., 2008. Epidemiology, etiology, pathogenesis, and diagnosis of recurrent bacterial meningitis. Clin Microbiol Rev, 21, 519–37. https://doi.org/10.1128/cmr.00009-08
[2]. Sundaram, S., Devanathan, V., Kanna, V., Kumar, S., Jayaraman, S., 2023. Etiological profile of new onset seizures above 60 years of age. J Indian Med Assoc, 121(5), 36-40.
[3]. Premavathy, D., 2020. Morphometric Analysis of occipital triangle in south Indian dry skulls. International Journal of Pharmaceutical Research (09752366), 12(4).
[4]. Gayatri Devi, R., Gayathri, R., 2020. Knowledge And Awareness on HIV and its impact on brain among dental students-a survey. International Journal of Pharmaceutical Research (09752366).
[5]. Xie, M., Zhou, K., Kachra, S., McHugh, T., Sommer, D. D., 2022. Diagnosis and localization of cerebrospinal fluid rhinorrhea: A systematic review. Am J Rhinol Allergy, 36, 397–406. https://doi.org/10.1177/19458924211060918
[6]. Thigpen, M. C, Whitney, C.G., Messonnier, N.E., Zell, E.R., Lynfield, R., Hadler, J.L., et al., 2011. Bacterial meningitis in the United States, 1998–2007. N Engl J Med, 364, 2016–25. https://doi.org/10.1056/nejmoa1005384
[7]. Adriani, K. S., van de Beek, D., Brouwer, M. C., Spanjaard, L., de Gans, J., 2007. Community-acquired recurrent bacterial meningitis in adults. Clin Infect Dis, 45, e46–51. https://doi.org/10.1086/520682
[8]. Huff, T., Tadi, P., Weisbrod, L. J., Varacallo, M., 2023. Neuroanatomy, cerebrospinal fluid. statpearls publishing; https://www.ncbi.nlm.nih.gov/books/NBK470578/
[9]. Mathias, T. L., Levy, J., Fatakia, A., McCoul, E. D., 2016, Contemporary approach to the diagnosis and management of Cerebrospinal fluid rhinorrhea. Ochsner J, 16, 136–42. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4896656/
[10]. Guyer, R. A., Turner, J. H., 2015, Delayed presentation of traumatic cerebrospinal fluid rhinorrhea: Case report and literature review. Allergy Rhinol (Providence) 6, ar.2015.6.0132. https://doi.org/10.2500/ar.2015.6.0132
[11]. Iffenecker, C., Benoudiba, F., Parker, F., Fuerxer, F., David, P., Tadie, M., et al., 1999, The place of MRI in the study of cerebrospinal fluid fistulas. J Radiol, 80, 37-43. https://pubmed.ncbi.nlm.nih.gov/10052036/
[12]. Bennett, N. M., Whitney, C.G., Moore, M., Pilishvili, T., Dooling, K. L., 2012. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP).
[13]. Wen, W., Xu, G., Zhang, X., Shi, J., Xie, M., Li, Y., et al., 2002. Surgical management of cerebrospinal fluid rhinorrhea. Zhonghua Er Bi Yan Hou Ke Za Zhi, 37, 366–9. https://pubmed.ncbi.nlm.nih.gov/12772459/
Viewed PDF 201 8 -
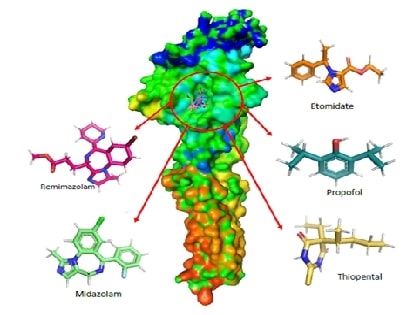 In-Silico Molecular Interaction and Pharmacokinetic Evaluation of Remimazolam and Major Intravenous Anesthetics Targeting GABAA ReceptorsAuthor: Jaganathan RamakrishnanDOI: 10.21522/TIJPH.2013.SE.24.05.Art011
In-Silico Molecular Interaction and Pharmacokinetic Evaluation of Remimazolam and Major Intravenous Anesthetics Targeting GABAA ReceptorsAuthor: Jaganathan RamakrishnanDOI: 10.21522/TIJPH.2013.SE.24.05.Art011In-Silico Molecular Interaction and Pharmacokinetic Evaluation of Remimazolam and Major Intravenous Anesthetics Targeting GABAA Receptors
Abstract:
This study investigates the molecular interactions and pharmacokinetic properties of five intravenous anaesthetics Remimazolam, Midazolam, Propofol, Thiopental, and Etomidate with the γ-Aminobutyric acid type A (GABAA) receptor, a key mediator of inhibitory neurotransmission in the central nervous system. Using molecular docking analysis, we evaluated the binding affinities of these drugs to the GABAA neurotransmitter receptor. Remimazolam emerged as a promising candidate with a docking score of -6.9 kcal/mol, demonstrating strong and stable interactions with critical receptor residues such as THR96 and GLN65. Although Midazolam exhibited a slightly superior docking score of -7.1 kcal/mol, Remimazolam’s pharmacokinetic profile offers distinct advantages, including rapid onset, short duration of action, and a favourable safety profile with minimal risk of hepatotoxicity and skin sensitization. In comparison, Propofol, Thiopental, and Etomidate showed weaker binding affinities and raised safety concerns. These findings suggest that Remimazolam is a competitive and safer alternative to existing intravenous anaesthetics, particularly in outpatient settings and procedures requiring efficient anaesthetic management. This study contributes valuable insights into the clinical application of Remimazolam, reinforcing its potential as an effective choice in the realm of intravenous anaesthesia.
In-Silico Molecular Interaction and Pharmacokinetic Evaluation of Remimazolam and Major Intravenous Anesthetics Targeting GABAA Receptors
References:
[1]. Weir, C. J., Mitchell, S. J., Lambert, J. J., 2017, Role of GABAA receptor subtypes in the behavioural effects of intravenous general anaesthetics. Br J Anaesth. 119: i167–i175. Doi:10.1093/bja/aex369.
[2]. Ramasamy, K., Shanmugasundaram, J., Manoharan, R., Subramanian, V., Kathirvelu, P., Vijayaraghavan, R., 2022, Anti-neuropathic effect of 7,3′-dihydroxyflavone in paclitaxel induced peripheral neuropathy in mice involving GABAA, KATP channel and adenosine receptors. Neurochem Int. 159: 105388. Doi: 10.1016/j.neuint.2022.105388.
[3]. Marimuthu M., 2021, Dental impactions performed under general anaesthesia - A retrospective study on the frequency and implications, Int J Dent Oral Sci. 1793–1796. Doi:10.19070/2377-8075-21000355.
[4]. Keerthika S, Mani G., 2021, Knowledge, attitude and practice of dentists towards dental procedures under general Anesthesia in children. J Pharm Res Int. 83–93. Doi:10.9734/jpri/2021/v33i20B31361.
[5]. Manivasagam D, Muthukrishnan A, Chaudary M., 2020, Assessment of effectiveness of local anesthesia with and without adrenaline in patients with cardiac disorders. Int J Pharm Res. 13. Doi:10.31838/ijpr/2021.13.01.217.
[6]. Dessai S, Ninave S, Bele A., 2024, The Rise of remimazolam: A Review of pharmacology, clinical efficacy, and safety profiles. Cureus. 16: e57260. Doi:10.7759/cureus.57260.
[7]. Kilpatrick G. J., 2021, Remimazolam: Non-Clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 12: 690875. Doi:10.3389/fphar.2021.690875.
[8]. Noor N, Legendre R, Cloutet A, Chitneni A, Varrassi G, Kaye A. D., 2021, A comprehensive review of remimazolam for sedation. Heal Psychol Res.;9: 24514. Doi:10.52965/001c.24514.
[9]. Dao V-A, Schippers F, Stöhr T., 2022, Efficacy of remimazolam versus midazolam for procedural sedation: Post hoc integrated analyses of three phase 3 clinical trials. Endosc Int open. 10: E378–E385. Doi:10.1055/a-1743-1936.
[10]. Dong L, Sun T, Yang J, Zhou Y, Liu X, Liu Z, et al., 2024, Remimazolam has similar anesthetic effect and superior safety compared to propofol in elderly patients: A meta‐analysis of randomized controlled trials. World J Surg. Doi:10.1002/wjs.12273.
[11]. Hoshino R, Ohashi N, Uta D, Ohashi M, Deguchi H, Baba H., 2024, Actions of remimazolam on inhibitory transmission of rat spinal dorsal horn neurons. J Pharmacol Sci. 155: 63–73. Doi: 10.1016/j.jphs.2024.04.002.
[12]. Masui K., 2024, Remimazolam: Its clinical pharmacology and evolving role in anesthesia and sedation practice. Curr Opin Anaesthesiol. 37: 344–351. Doi:10.1097/ACO.0000000000001384.
[13]. Kim, K. M., 2022, Remimazolam: Pharmacological characteristics and clinical applications in anesthesiology. Anesth pain Med. 17: 1–11. Doi:10.17085/apm.21115
[14]. Alharbi K. S, Almalki W. H, Alzarea S. I, Kazmi I, Al-Abbasi F. A, Afzal O, et al., 2024, Anaesthesia-induced changes in genomic expression leading to neurodegeneration. CNS Neurol Disord - Drug Targets. 23: 411–419. Doi:10.2174/1871527322666230508123558.
[15]. Mahmoud M, Mason KP., 2018, Recent advances in intravenous anesthesia and anesthetics. F1000Research. 7: 470. Doi:10.12688/f1000research.13357.1
[16]. Berman H. M, Battistuz T, Bhat T. N, Bluhm W. F, Bourne P. E, Burkhardt K, et al., 2002, The protein data bank. Acta Crystallogr Sect D Biol Crystallogr. 58: 899–907. Doi:10.1107/S0907444902003451.
[17]. Miller P. S, Aricescu A. R., 2014, Crystal structure of a human GABAA receptor. Nature.;512: 270–275. Doi:10.1038/nature13293.
[18]. Zielesny A., 2005, Chemistry software package chemoffice ultra 2005. J Chem Inf Model. 45: 1474–1477. Doi:10.1021/ci050273j.
[19]. Cousins K., 1993, ChemOffice Plus: A package of programs for chemists. J Chem Inf Comput Sci. 33: 788–789. Doi:10.1021/ci00015a603.
[20]. Morris G. M, Huey R, Lindstrom W, Sanner M. F, Belew R. K, Goodsell D. S, et al., 2010, AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 30: 2785–2791. Doi: 10.1002/jcc.21256.AutoDock4
[21]. The PyMOL Molecular Graphics System, Version 2.0, Schrödinger, LLC. 2002. Available: https://pymol.org
[22]. SYSTÈMES D. BIOVIA Discovery Studio. Dassault Syst mes BIOVIA, Discovery Studio Modeling Environment, Release 2017. Dassault Syst mes; 2016. Available: http://accelrys.com/products/collaborative-science/biovia-discovery-studio/
[23]. Pires D. E V., Blundell T. L, Ascher D. B., 2015, pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem. 58: 4066–4072. Doi: 10.1021/acs.jmedchem.5b00104.
[24]. Jia C-Y, Li J-Y, Hao G-F, Yang G-F., 2020, A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov Today. 25: 248–258. Doi: 10.1016/j.drudis.2019.10.014.
[25]. Kesharwani R. K, Vishwakarma V. K, Keservani R. K, Singh P, Katiyar N, Tripathi S., 2020, Role of ADMET tools in current scenario: Application and limitations. Computer-Aided Drug Design. Singapore: Springer Singapore. pp. 71–87. Doi:10.1007/978-981-15-6815-2_4.
Viewed PDF 227 11 -
 A Brief Narrative Review on Prevalence of Diabetes in Tamil NaduAuthor: Vijayanchali S SDOI: 10.21522/TIJPH.2013.SE.24.05.Art012
A Brief Narrative Review on Prevalence of Diabetes in Tamil NaduAuthor: Vijayanchali S SDOI: 10.21522/TIJPH.2013.SE.24.05.Art012A Brief Narrative Review on Prevalence of Diabetes in Tamil Nadu
Abstract:
The incidence of diabetes in Tamil Nadu has escalated significantly in recent decades, mirroring a wider national pattern observed throughout India. This narrative review synthesizes an extensive array of literature concerning the epidemiology, risk factors and health consequences associated with diabetes in Tamil Nadu, illuminating the complex interplay of various contributing elements. A key focus of this analysis is the examination of how genetic susceptibility interacts with rapid urban development, lifestyle modifications and socio-economic factors, culminating in a scenario that exacerbates the rising diabetes rates in the region. The review also investigates regional disparities, revealing marked differences in diabetes prevalence between urban and rural areas, as well as among diverse demographic segments, including age, gender and occupation. These disparities highlight the necessity for localized evaluations to enhance the understanding of diabetes distribution in varied contexts. Furthermore, the review identifies critical obstacles to effective diabetes management and prevention in Tamil Nadu such as limited access to healthcare, low public awareness and inadequate health promotion initiatives, which collectively impede efforts to address the diabetes epidemic. By scrutinizing these elements this review aspires to enrich the understanding of diabetes in Tamil Nadu and emphasizes the urgent need for targeted interventions that cater to the unique challenges faced by different communities. Such insights are vital for shaping public health strategies aimed at alleviating the escalating diabetes crisis in Tamil Nadu and fostering improved health outcomes throughout the state.
A Brief Narrative Review on Prevalence of Diabetes in Tamil Nadu
References:
[1] Geetha, R. V., 2020, Diabetes mellitus related knowledge and awareness-a survey among dental students. The Journal of Contemporary Issues in Business and Government, 26(2), 1582–1594.
[2] Ramamurthy, J., 2023, Prevalence of oral mucosal lesions in patients with type II diabetes mellitus: A retrospective analysis. World Journal of Dentistry, 14(8), 683–687.
[3] Jitesh, S., Rajasekar, A., & Madhulaxmi, M., 2021, Prevalence of periodontitis in patients with controlled and uncontrolled diabetes mellitus. Int J Dentistry Oral Sci, 8(8), 4070–4073.
[4] Samyuktha, P. S., & Syam, S., 2024, Periodontal abscess as a clinical oral sign in patients with diabetes mellitus-an original study. Bulletin of Pioneering Researches of Medical and Clinical Science, 3(2–2024), 7–12.
[5] Sowmya, S., & Sangavi, R., 2024, Effectiveness of oral health education and interventions in improving oral health outcomes in Type II diabetes mellitus patients: A prospective study. Cureus, 16(4).
[6] Govindaswamy, S., & Dhivya, P. S., 2022, Prevalence and complications of diabetes mellitus in India-A systematic review.
[7] Kumar, A., Gangwar, R., Ahmad Zargar, A., Kumar, R., & Sharma, A., 2024, Prevalence of diabetes in India: A review of IDF diabetes atlas 10th edition. Current Diabetes Reviews, 20(1), 105–114.
[8] Kumar, A., Gangwar, R., Ahmad Zargar, A., Kumar, R., & Sharma, A., 2024, Prevalence of diabetes in India: A review of IDF diabetes atlas 10th edition. Current Diabetes Reviews, 20(1), 105–114.
[9] Faiz, N., & Rajaraman, V., 2024, Estimation of inflammatory markers IL-1 And IL-6 level in completely edentulous diabetic patients. African Journal of Biomedical Research, 27(1S), 268–272.
[10] Smokovski, I., & Smokovski, I., 2021, Burden of diabetes prevalence. Managing diabetes in low income countries: providing sustainable diabetes care with limited resources, 1–12.
[11] Mohan, V., Unnikrishnan, R., & Anjana, R. M., 2023, Comment on Rooney et al. Global prevalence of prediabetes. Diabetes Care 2023; 46: 1388–1394. Diabetes Care, 46(12), e220–e220.
[12] Kumaran, K. M., Vedapriya, D. R., Manoharan, A., & Nirupama, A. Y., 2023, Indian diabetic risk score screening of rural adults in Tamil Nadu. IHOPE Journal of Ophthalmology, 2(2), 31–35.
[13] Prasath, R., & Sinduja, P., 2023, Knowledge and Awareness on Various Treatment Modalities of Diabetes Mellitus-A Observational Survey.
[14] Selvavinayagam, T. S., Viswanathan, V., Ramalingam, A., Kangusamy, B., Joseph, B., Subramaniam, S., Sheela, J. S., Wills, S., Ramasamy, S., & Venkatasamy, V., 2024, Prevalence of Noncommunicable Disease (NCDs) risk factors in Tamil Nadu: Tamil Nadu STEPS Survey (TN STEPS), 2020. Plos One, 19(5), e0298340.
[15] Kalaiselvi, S., Syed Ali, M., Anuradha, V., & Subhashini, A., 2023, A study on the association of diabetes and semen quality in and around Chennai, Tamil Nadu, India. International Journal of Reproduction, Contraception, Obstetrics and Gynecology, 12(4), 911.
[16] Tamilarasan, M., Kulothungan, K., Rizvana, S., & Thirunavukkarasu, S., 2023, A cross-sectional study of determinants of type 2 diabetes mellitus among professional drivers in the perambalur municipality area of Tamil Nadu, India. Cureus, 15(1).
[17] Prasanth, B. K., Eashwar, V. M. A., Mahalakshmi, K., & Ramachandran, K., 2023, Epidemiology of non-communicable diseases among transgender population residing in Chennai district, Tamil Nadu. Journal of Family Medicine and Primary Care, 12(4), 762–767.
[18] Anusuya, G. S., Ravi, R., Gopalakrishnan, S., Abiselvi, A., & Stephen, T., 2018, Prevalence of undiagnosed and uncontrolled diabetes mellitus among adults in South Chennai. Int J Community Med Public Health, 5(12), 5200–5204.
[19] Runtu, A. R., Enggune, M., Pondaag, A., Lariwu, C., Sarayar, C., Pondaag, L., Lolowang, N., Merentek, G., Lontaan, E., & Sumarauw, J., 2024, Penyuluhan KESEHATAN DIABETES MELLITUS dan Deteksi Kadar Gula Darah pada Lansia. Jurnal Pengabdian Kepada Masyarakat Nusantara, 5(1), 1492–1499.
[20] Varghese, J. S., & Ali, M. K., 2022, Diabetes prevalence, screening, diagnosis, treatment and control in India: A cross-sectional study of individuals aged 18 years and older. Circulation, 146(Suppl_1), A13817–A13817.
[21] Govindaswamy, S., & Dhivya, P. S., 2022, Prevalence and complications of diabetes mellitus In India-A systematic review.
[22] Premavathy, D., 2021, Survey on problems faced by dental patients having diabetes mellitus. Journal of Pharmaceutical Research International, 33(64B), 106–113.
[23] Theivasigamani, K., & Palaniappan, S. Kumar., 2023, Prevalence and incidence of type-2 diabetes mellitus among the rural population of Erode district of Tamil Nadu, South India.
[24] Divya, P. V, & Sukesh, K., 2023, Assessment of knowledge and prevalence of urinary tract infections among Kanyakumari population. Hindu, 800(365), 45–62.
[25] Govindaswamy, S., & Dhivya, P. S., 2022, Prevalence and complications of diabetes mellitus In India-A systematic review.
[26] Jabbar, P. K., Nair, A., Chellamma, J., Jayakumar, R. V, Ramesh, J., Gomez, R., Vishnu, G. G., Voise, S., Soumya, S., & Vijayakumar, K., 2023, Type 2 Diabetes and precursors in community dwelling asian Indian adult youth. Indian Journal of Endocrinology and Metabolism.
[27] Raghavi, S. S., Eashwar, V. M. A., Dutta, R., Jain, T., & Eashwar, V. M. A., 2023, A study on morbidity profile of residents of an old age home in an Urban Area of Kancheepuram District, Tamil Nadu.
[28] Sandhya, M., Manikumar, M., Augustina, S. J., & Kamalakannan, M., 2022, Prevalence of diabetes mellitus and depression status among pregnant women in rural population in Kancheepuram District. Journal of Datta Meghe Institute of Medical Sciences University, 17(4), 871–876.
[29] Priyadharshini, S. N., & Bhuvaneswari, K. N., 2024, Study on prevalence of glaucoma among adult patients attending ophthal-mology department in a tertiary care hospital, Kanchipuram. J Clin Images Med Case Rep, 5(3), 2947.
[30] Thendralan, V., & Priya, S., 2024, IJCM_315A: Pesticide exposure and diabetes mellitus-A community based analytical cross-sectional study among agricultural workers in Madurai east community block, Tamil Nadu. Indian Journal of Community Medicine, 49 (Suppl 1), S91.
[31] Santhosh, M., Juliet, A. H., & Nataraj, C., 2024, Novel prediction of diabetes disease with improved accuracy by comparing K-means with logistic regression. AIP Conference Proceedings, 3161(1).
[32] Sathiyabama, G., & Mareeshwaran, S., 2024, Efficacy of certain nursing interventions on neuropathy aching Among patients with diabetic mellitus. 2024 International Conference on Intelligent Systems for Cybersecurity (ISCS), 1–6.
[33] Mohanraj, S., Velmurugan, G., Dhivakar, M., Ramakrishnan, A., Cherian, M., Alexander, T., Thalappil, P., & Swaminathan, K., 2022, Gender differential prevalent of overweight and obesity, hypertension and diabetes in South India: A population-based cross-sectional study.
[34] Kandasamy, K., Rajagopal, S. S., Ramalingam, K., & Krishnan, K., 2018, Prevalence of diagnosed and undiagnosed diabetes in a rural community: A home-based screening. Prevalence, 11(5).
[35] Chu, H., Tamous, S., Kavvi, A., & Khan, A., 2024, Diabetes Health Education for South Jersey Communities.
[36] Ali, J. B. A., Mohan, R., & Dinesh, T., 2024, Association between coronary artery disease and body mass index. www.Ijphrd.com, 15(1), 392.
[37] Varghese, J. S., & Ali, M. K., 2022, Diabetes prevalence, screening, diagnosis, treatment and control in India: A cross-sectional study of individuals aged 18 Years and Older. Circulation, 146(Suppl_1), A13817–A13817.
[38] Williams, H., & Ranganathan, S., 2024, A cross-sectional study on knowledge, attitude, and practice among type 2 diabetes mellitus patients attending a primary health care center in the rural region of Tamil Nadu.
[39] Russo, M. P., Grande-Ratti, M. F., Burgos, M. A., Molaro, A. A., & Bonella, M. B., 2023, Prevalence of diabetes, epidemiological characteristics and vascular complications. Archivos de Cardiología de México, 93(1), 30–36.
Viewed PDF 390 9 -
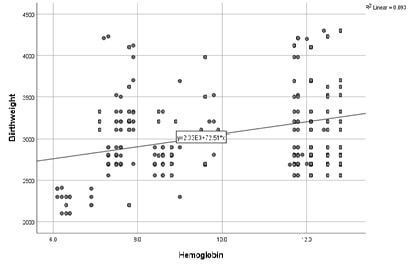 The Effect of Maternal Anaemia on Infant Birth Weight: A Comprehensive StudyAuthor: Vinod Kumar NelsonDOI: 10.21522/TIJPH.2013.SE.24.05.Art013
The Effect of Maternal Anaemia on Infant Birth Weight: A Comprehensive StudyAuthor: Vinod Kumar NelsonDOI: 10.21522/TIJPH.2013.SE.24.05.Art013The Effect of Maternal Anaemia on Infant Birth Weight: A Comprehensive Study
Abstract:
The objective of the present study was to understand about the maternal anaemia on the low birth weight of the neonates and also how it effects the maternal health. This study was conducted at the postnatal ward of the Saveetha Medical College, Chennai, during the period of December 2022 and July 2023. A total of 237 deliveries were analysed for this study. Maternal anaemia is divided in four categories based on the haemoglobin levels: no anaemia (>10 gm%), mild (8-10 gm%), moderate (7-8 gm%), severe (<7 gm%). Neonates were assessed for the birth weight and other perinatal factors. Statistical analysis for this study is done by using the chi-square and correlation coefficients. C Among the 236 antenatal women included, 49.2% were found to be anaemic. The average haemoglobin level was 10.05 ± 2.27 g/dL, and the average birth weight was 3055.81 ± 540.63 g. Of the infants, 8.1% were classified as LBW. A significant correlation was found between maternal anaemia and LBW: 37.9% of LBW infants were born to mothers with severe anaemia (p < 0.001). A statistically significant weak correlation (r = 0.305, p < 0.001) was observed between haemoglobin levels and neonatal weight.
The Effect of Maternal Anaemia on Infant Birth Weight: A Comprehensive Study
References:
[1]. Najeeba, C. M., Prabhu, A. S., Saldanha, P. R. M., 2015, Maternal anaemia and its effect on cord blood haemoglobin and newborn birth weight. IOSR, (14), 7:30-2.
[2]. Reddy, V., Rao, N. P., Sastry, J. G., Kashinath, K., 1993, Nutrition trends in India. Hyderabad National Institute of Nutrition, 1-108.
[3]. Dutta, D. C., 2013, Text book of obstetrics. 7th ed. Culcutta: New Central Book Agency, 457-461.
[4]. Milman, N., 2008, Prepartum anaemia: prevention and treatment. Ann Hematol, 87(12),949-59.
[5]. Singla, P. N., 1978, Chand, S.K., Hanna, S. U., Agarwal, K. N., Effect of maternal anaemia on the placenta and the newborn infant. Acta Paed, 67(5), 645-8.
[6]. Singla, P. N., Tyagi, M., Kumar, A., Dash, D., Shankar, R., 1997, Fetal growth in maternal anaemia. J Trop Pediatrics, 43(2),89-92.
[7]. Rusia, U. S., Madan, N. I., Agarwal, N. E., Sikka, M. E., Sood, S. K., 1995, Effect of maternal iron deficiency anaemia on foetal outcome. Ind J Pathol Microbiol, 38(3),273-9.
[8]. Nair, M., Gireesh, S., Yakoob, R., Cherian, N. C., 2018, Effect of maternal anaemia on birth weight of term babies Int J Contemp Pediatr, 5(3),1019-1022.
[9]. Shanmugam, R., Tharani, M., Abullais, S. S., 2024, Black seed assisted synthesis, characterization, free radical scavenging, antimicrobial and anti-inflammatory activity of iron oxide nanoparticles. BMC Complement Med Ther 24(24):241.
[10]. Habeeb Rahuman, H. B., Dhandapani, R., Narayanan, S., Palanivel, V., Paramasivam, R., Subbarayalu, R., Thangavelu, S., Muthupandian, S., 2022, Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnol. 16(4):115-144.
[11]. Wadhwa, R., Paudel, K. R., Chin, L. H., Hon, C. M., Madheswaran, T., Gupta, G., Panneerselvam, J., Lakshmi, T., 2021, Anti-inflammatory and anticancer activities of Naringenin-loaded liquid crystalline nanoparticles in vitro. J Food Biochem. 45(1): e13572.
Viewed PDF 188 8 -
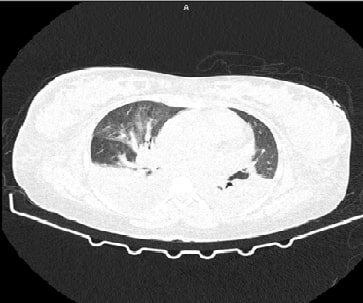 The Spectrum of RHD Management in Pregnancy on Assessing the Time of Diagnosis, Severity of Cardiac Lesions, and the ChallengesAuthor: Maghimaa MDOI: 10.21522/TIJPH.2013.SE.24.05.Art014
The Spectrum of RHD Management in Pregnancy on Assessing the Time of Diagnosis, Severity of Cardiac Lesions, and the ChallengesAuthor: Maghimaa MDOI: 10.21522/TIJPH.2013.SE.24.05.Art014The Spectrum of RHD Management in Pregnancy on Assessing the Time of Diagnosis, Severity of Cardiac Lesions, and the Challenges
Abstract:
Rheumatic heart disease (RHD) poses a significant challenge during pregnancy, especially in low- and middle-income countries, where it affects millions of individuals, primarily young women of childbearing age. This autoimmune condition, often triggered by group A streptococcal infections, leads to chronic inflammation and scarring of heart valves, resulting in insufficiency and stenosis. Pregnancies complicated by cardiovascular diseases, including RHD, contribute to maternal mortality rates and present unique management dilemmas due to the physiological changes accompanying gestation. This case series presents three cases illustrating the complexities of managing RHD during pregnancy. Case 1 describes a gravid woman with a history of previous normal delivery and hepatitis B surface antigen positivity, requiring emergency intervention due to fetal distress and elevated blood pressure. Case 2 details the management of RHD in a woman with hypothyroidism presenting with labor pain at term gestation. Case 3 presents the challenges of managing RHD in a primigravida with a history of nephroureterectomy for ectopic ureter insertion. Understanding the pathological mechanisms and tailored management strategies for pregnant women with RHD is crucial for improving care and outcomes.
The Spectrum of RHD Management in Pregnancy on Assessing the Time of Diagnosis, Severity of Cardiac Lesions, and the Challenges
References:
[1]. Rich-Edwards, J. W., Fraser, A., Lawlor, D. A., Catov, J. M., 2014, Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women's health? Epidemiol Rev, 36:57–70. https://doi.org/10.1093/epirev/mxt006
[2]. Nanna, M., Stergiopoulos, K., 2014, Pregnancy complicated by valvular heart disease: An update. J Am Heart Assoc. 3: e000712. https://doi.org/10.1161/JAHA.113.000712
[3]. Gongora, M. C., Wenger, N. K., 2015, Cardiovascular complications of pregnancy. Int J Mol Sci. 16:23905-28. https://doi.org/10.3390/ijms161023905
[4]. Lameijer, H., Burchill, L. J., Baris, L., Ruys, T. P., Roos-Hesselink, J. W., Mulder, B. J. M., et al., 2019, Pregnancy in women with pre-existent ischaemic heart disease: A systematic review with individualised patient data. Heart. 105:873–80. https://doi.org/10.1136/heartjnl-2018-314364
[5]. Khairy, P., Ouyang, D. W., Fernandes, S. M., Lee-Parritz, A., Economy, K. E., Landzberg, M, J., 2006, Pregnancy outcomes in women with congenital heart disease. Circulation. 113:517-24. https://doi.org/10.1161/CIRCULATIONAHA.105.589655
[6]. Siu, S. C., Sermer, M., Colman, J. M., Alvarez, A. N., Mercier, L. A., Morton, B. C., et al., 2001, Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 104:515–21. https://doi.org/10.1161/hc3001.093437
[7]. Sengodan, S. S., Selvara, j S., 2019, Study of prevalence of heart disease in antenatal mothers along with fetal and maternal outcome from a tertiary care hospital, Salem, Tamil Nadu, India. International Journal of Reproduction, Contraception, Obstetrics and Gynecology. 8:5027.
[8]. Konar, H., Chaudhuri, S., 2012, Pregnancy complicated by maternal heart disease: A review of 281 women. J Obstet Gynaecol India. 62:301–6. https://doi.org/10.1007/s13224-012-0220-2
[9]. Aye, C. Y., Boardman, H., Leeson, P., 2017, Cardiac disease after pregnancy: A Growing Problem. Eur Cardiol. 12:20–3. https://doi.org/10.15420/ecr.2017:4:1
[10]. Zühlke, L., Engel, M. E., Karthikeyan, G., Rangarajan, S., Mackie, P., Cupido, B., et al., 2015, Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. 36:1115–22a. https://doi.org/10.1093/eurheartj/ehu449
[11]. Shapiro, H., Alshawabkeh, L., 2024, Valvular heart disease in pregnancy. Methodist Debakey Cardiovasc J. 20:13–23. https://doi.org/10.14797/mdcvj.1323
[12]. Chhetri, S., Shrestha, N. R., Pilgrim, T., 2014, Pregnancy complicated by heart disease in Nepal. Heart Asia. 6:26–9. https://doi.org/10.1136/heartasia-2013-010396
[13]. Khanna, R., Chandra, D., Yadav, S., Sahu, A., Singh, N., Kumar, S., et al., 2021, Maternal and fetal outcomes in pregnant females with rheumatic heart disease. Indian Heart J. 73:185–9. https://doi.org/10.1016/j.ihj.2021.01.012
[14]. Fraccaro, C., Tence, N., Masiero, G., Karam, N., 2020, Management of valvular disease during pregnancy: Evolving role of percutaneous treatment. Interv Cardiol. 15:e10. https://doi.org/10.15420/icr.2020.06
[15]. Patel, C., Akhtar, H., Gupta, S., Harky, A., 2020, Pregnancy and cardiac interventions: What are the optimal management options? J Card Surg. 35:1589–96. https://doi.org/10.1111/jocs.14637
[16]. Bhatla, N., Lal, S., Behera G, Kriplani A, Mittal S, Agarwal N, et al., 2003, Cardiac disease in pregnancy. Int J Gynaecol Obstet. 82:153–9. https://doi.org/10.1016/s0020-7292(03)00159-0
[17]. Sharma, B., Koirala, E., Regmi, S., Dhungana, J., Neupane, B. K., Bhattarai, S., 2021, Rheumatic heart disease among pregnant women with cardiac diseases in a tertiary care center of nepal: A descriptive cross-sectional study. JNMA J Nepal Med Assoc. 59:468–72. https://doi.org/10.31729/jnma.6177
[18]. Naik, A. S., Naik, S. A., Shinde, S., Vaje, M. C. 2022, A retrospective study to evaluate the maternal and fetal outcome in patients of heart disease in pregnancy at a tertiary rural hospital. Journal of Medical Sciences, 8(2), 113.
[19]. Aishwarya, R., Ethirajan, S., 2022, Gestational age at booking for antenatal care in a tertiary healthcare facility: a glance. International Journal of Infertility & Fetal Medicine, 13(3), 91-95.
[20]. Jaya Keerthana, S., Maragathavalli, G., 2021, Prevalence of oral lesions in pregnant patients. International Journal of Dentistry and Oral Science, 8(7), 3105 – 3107.
[21]. Ethirajan, S., Pritem, M. L., 2020. Study on knowledge and practice of periconceptional intake of folic acid among antenatal mothers at Saveetha Medical College Hospital, Tamil Nadu. Age, 20(25), 12-5.
Viewed PDF 187 3 -
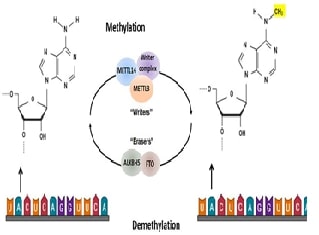 M6A RNA Methylation and Cancer Progression: Pathogenesis, Implications, and Therapeutic PotentialAuthor: Geetha ShanmugamDOI: 10.21522/TIJPH.2013.SE.24.05.Art015
M6A RNA Methylation and Cancer Progression: Pathogenesis, Implications, and Therapeutic PotentialAuthor: Geetha ShanmugamDOI: 10.21522/TIJPH.2013.SE.24.05.Art015m6A RNA Methylation and Cancer Progression: Pathogenesis, Implications, and Therapeutic Potential
Abstract:
N6-methyladenosine (m6A) is a prominent internal modification of eukaryotic messenger RNA (mRNA) and has garnered significant attention due to its regulatory role in various biological processes, particularly in cancer. As a dynamic and reversible modification, m6A is orchestrated by three key players: Writers, primarily methyltransferases like METTL3 and METTL14, add m6A marks to mRNA, influencing processes such as RNA stability and translation. Erasers, such as FTO and ALKBH5, remove these marks, enabling the fine-tuning of mRNA functions. Readers, including YTH domain-containing proteins (e.g., YTHDF1, YTHDF2), recognize and bind to m6A-modified mRNA, directing its fate in terms of degradation, translation efficiency, or cellular localization. In cancer, m6A modifications have been implicated in promoting tumor progression by affecting gene expression at the post-transcriptional level. The dysregulation of m6A machinery can lead to aberrant expression of oncogenes or tumor suppressors, thereby driving oncogenesis Furthermore, m6A modifications play a crucial role in modulating the tumor microenvironment (TME) and immune evasion. By altering the expression of cytokines, chemokines, and immune checkpoint molecules, m6A can influence immune cell infiltration and activation, impacting the anti-tumor immune response. This modulation of the TME not only aids in tumor progression but also presents challenges and opportunities for cancer immunotherapy. Given its profound impact on cancer biology, targeting the m6A machinery holds therapeutic potential. Small molecules or inhibitors designed to modulate m6A writers, erasers, or readers could offer new avenues for cancer treatment, making m6A a promising target in the fight against cancer.
m6A RNA Methylation and Cancer Progression: Pathogenesis, Implications, and Therapeutic Potential
References:
[1]. Ji, P., Wang, X., Xie, N., & Li, Y., 2018, N6-Methyladenosine in RNA and DNA: An epitranscriptomic and epigenetic player implicated in determination of stem cell fate. Stem Cells International, 2018, 3256524., https://doi.org/10.1155/2018/3256524
[18]. Quan, C., Belaydi, O., Hu, J., Li, H., Yu, A., Liu, P., Yi, Z., Qiu, D., Ren, W., Ma, H., Gong, G., Ou, Z., Chen, M., Sun, Y., Chen, J., & Zu, X., 2021, N6-Methyladenosine in cancer immunotherapy: an undervalued therapeutic target. Frontiers in Immunology, 12, 697026. https://doi.org/10.3389/fimmu.2021.697026
[20]. Zhou, H., Sun, Q., Feng, M., Gao, Z., Jia, S., Cao, L., Yu, X., Gao, S., Wu, H., & Li, K., 2023, Regulatory mechanisms and therapeutic implications of insulin-like growth factor 2 mRNA-binding proteins, the emerging crucial m6A regulators of tumors. Theranostics, 13(12), 4247–4265. https://doi.org/10.7150/thno.86528
[27]. Meijing, Z., Tianhang, L., & Biao, Y. 2022, N6-Methyladenosine modification patterns and tumor microenvironment immune characteristics associated with clinical prognosis analysis in stomach adenocarcinoma. Frontiers in Cell and Developmental Biology, 10, 913307. https://doi.org/10.3389/fcell.2022.913307
[28]. Arumugam, P., Jayaseelan V. P., 2022, 15P N6-Methyladenosine modification of YY1 mRNA promotes cervical cancer tumorigenesis. Annals of Oncology, 33, 388. https://www.annalsofoncology.org/article/S0923-7534(22)00718-9/fulltext
[29]. Krishnamoorthy, H. S., Kannan, B., Ganapathy, D., Jayaseelan, V. P., & Arumugam, P., 2023, Decreased expression of the m6A RNA methyltransferase METTL3 is associated with residual ridge resorption. Journal of Oral Biology and Craniofacial Research, 13(5), 563–566. https://doi.org/10.1016/j.jobcr.2023.07.003
[30]. Krishnamoorthy, H. S., Kannan, B., Ganapathy, D., Jayaseelan, V. P., & Arumugam, P. 2023, Dysregulated m6A methylation modification is associated with human peri-implantitis - A pilot study. Journal of Stomatology, Oral and Maxillofacial Surgery, 124(6S), 101550. https://doi.org/10.1016/j.jormas.2023.101550
Viewed PDF 203 1 -
 An Update on the Role of Omega-3 Fatty Acids in Metabolic Health and Insulin Resistance: A narrative reviewAuthor: Monisha PrasadDOI: 10.21522/TIJPH.2013.SE.24.05.Art016
An Update on the Role of Omega-3 Fatty Acids in Metabolic Health and Insulin Resistance: A narrative reviewAuthor: Monisha PrasadDOI: 10.21522/TIJPH.2013.SE.24.05.Art016An Update on the Role of Omega-3 Fatty Acids in Metabolic Health and Insulin Resistance: A narrative review
Abstract:
Insulin resistance and metabolic health are largely regulated by important pathways that omega-3 fatty acids influence. The molecular pathways by which Omega-3 fatty acids, specifically Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA), work is the main topic of concern in this narrative overview. Through modifying inflammatory responses, enhancing lipid metabolism, and impacting insulin signaling pathways, these fatty acids mainly increase insulin sensitivity. Studies have demonstrated that EPA and DHA can increase insulin sensitivity by downregulating the nuclear factor kappa B (NF-κB) pathway, reducing pro-inflammatory cytokines, and increasing the activation of peroxisome proliferator-activated receptors (PPARs). Furthermore, Omega-3s mitigate lipotoxicity and encourage the effective utilization of fatty acids as an energy source, all of which are critical for preserving insulin sensitivity. They also lessen ectopic fat accumulation. Additionally, the interaction between Omega-3s and membrane phospholipids enhances insulin receptor signaling and activity. Notwithstanding these advantages, further research is necessary to fully understand the unique impacts of certain Omega-3s, such as Alpha-Linolenic Acid (ALA), on these pathways as well as how they might interact with other dietary components and gut flora. We can more effectively utilize the therapeutic potential of omega-3 fatty acids to enhance metabolic health and combat insulin resistance by clarifying these pathways.
An Update on the Role of Omega-3 Fatty Acids in Metabolic Health and Insulin Resistance: A narrative review
References:
[1]. Chatterjee, S., Khunti, K., & Davies, M. J., 2017, Type 2 diabetes. Lancet, 389(10085), 2239–2251, https://doi.org/10.1016/S0140-6736(17)30058-2
[2]. Rajendran, S., Mishra, S., Madhavanpillai, M., & G, V., 2022, Association of hemoglobin glycation index with cardiovascular risk factors in non-diabetic adults: A cross-sectional study. Diabetes & Metabolic Syndrome, 16(9), 102592, https://doi.org/10.1016/j.dsx.2022.102592
[3]. Sinha, S., Haque, M., Lugova, H., & Kumar, S., 2023, The effect of Omega-3 fatty acids on insulin resistance. Life, 13(6), 1322, https://doi.org/10.3390/life13061322
[4]. Natto, Z. S., Yaghmoor, W., Alshaeri, H. K., & Van Dyke, T. E., 2019, Omega-3 fatty acids effects on inflammatory biomarkers and lipid profiles among diabetic and cardiovascular disease patients: A systematic review and meta-analysis. Scientific Reports, 9(1), 18867, https://doi.org/10.1038/s41598-019-54535-x
[5]. Yaribeygi, H., Maleki, M., Jamialahmadi, T., Shakhpazyan, N. K., Kesharwani, P., & Sahebkar, A., 2023, Nanoparticles with SGLT2 inhibitory activity: Possible benefits and future. Diabetes & Metabolic Syndrome, 17(10), 102869, https://doi.org/10.1016/j.dsx.2023.102869
[6]. Samuel, V. T., Petersen, K. F., & Shulman, G. I., 2010, Lipid-induced insulin resistance: Unravelling the mechanism. Lancet, 375(9733), 2267–2277, https://doi.org/10.1016/S0140-6736(10)60408-4
[7]. Winn, N. C., Pettit-Mee, R., Walsh, L. K., Restaino, R. M., Ready, S. T., Padilla, J., & Kanaley, J. A., 2019, Metabolic implications of diet and energy intake during physical inactivity. Medicine and Science in Sports and Exercise, 51(5), 995–1005, https://doi.org/10.1249/MSS.0000000000001892
[8]. Rahman, M. S., Hossain, K. S., Das, S., Kundu, S., Adegoke, E. O., Rahman, M. A., Hannan, M. A., Uddin, M. J., & Pang, M. G., 2021, Role of insulin in health and disease: An update. International Journal of Molecular Sciences, 22(12), 6403, https://doi.org/10.3390/ijms22126403
[9]. Natesan, V., & Kim, S. J., 2021, Lipid metabolism, disorders and therapeutic drugs - Review. Biomolecules & Therapeutics, 29(6), 596–604, https://doi.org/10.4062/biomolther.2021.122
[10]. Hardy, O. T., Czech, M. P., & Corvera, S., 2012, What causes the insulin resistance underlying obesity? Current Opinion in Endocrinology, Diabetes, and Obesity, 19(2), 81–87, https://doi.org/10.1097/MED.0b013e3283514e13
[11]. Rekha, K., Venkidasamy, B., Samynathan, R., Nagella, P., Rebezov, M., Khayrullin, M., Ponomarev, E., Bouyahya, A., Sarkar, T., Shariati, M. A., Thiruvengadam, M., & Simal-Gandara, J., 2024, Short-chain fatty acid: An updated review on signaling, metabolism, and therapeutic effects. Critical Reviews in Food Science and Nutrition, 64(9), 2461–2489, https://doi.org/10.1080/10408398.2022.2124231
[12]. Cholewski, M., Tomczykowa, M., & Tomczyk, M., 2018, A comprehensive review of chemistry, sources and bioavailability of omega-3 fatty acids. Nutrients, 10(11), 1662, https://doi.org/10.3390/nu10111662
[13]. Rodriguez-Leyva, D., Dupasquier, C. M., McCullough, R., & Pierce, G. N., 2010, The cardiovascular effects of flaxseed and its omega-3 fatty acid, alpha-linolenic acid. The Canadian Journal of Cardiology, 26(9), 489–496, https://doi.org/10.1016/s0828-282x(10)70455-4
[14]. Weitz, D., Weintraub, H., Fisher, E., & Schwartzbard, A. Z., 2010, Fish oil for the treatment of cardiovascular disease. Cardiology in Review, 18(5), 258–263, https://doi.org/10.1097/CRD.0b013e3181ea0de0
[15]. Bradbury, J., 2011, Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients, 3(5), 529–554, https://doi.org/10.3390/nu3050529
[16]. Calder, P. C., 2010, Omega-3 fatty acids and inflammatory processes. Nutrients, 2(3), 355–374, https://doi.org/10.3390/nu2030355
[17]. Samanta, S., Sarkar, T., Chakraborty, R., Rebezov, M., Shariati, M. A., Thiruvengadam, M., & Rengasamy, K. R. R., 2022, Dark chocolate: An overview of its biological activity, processing, and fortification approaches. Current Research in Food Science, 5, 1916–1943, https://doi.org/10.1016/j.crfs.2022.10.017
[18]. Gharraee, N., Wang, Z., Pflum, A., Medina-Hernandez, D., Herrington, D., Zhu, X., & Meléndez, G. C., 2022, Eicosapentaenoic acid ameliorates cardiac fibrosis and tissue inflammation in spontaneously hypertensive rats. Journal of Lipid Research, 63(11), 100292, https://doi.org/10.1016/j.jlr.2022.100292
[19]. Chen, C., Yang, Y., Yu, X., Hu, S., & Shao, S., 2017, Association between omega-3 fatty acids consumption and the risk of type 2 diabetes: A meta-analysis of cohort studies. Journal of Diabetes Investigation, 8(4), 480–488, https://doi.org/10.1111/jdi.12614
[20]. Li, M., Chi, X., Wang, Y., Setrerrahmane, S., Xie, W., & Xu, H., 2022, Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduction and Targeted Therapy, 7(1), 216, https://doi.org/10.1038/s41392-022-01073-0
[21]. Qiu, Y. Y., Zhang, J., Zeng, F. Y., & Zhu, Y. Z., 2023, Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Pharmacological Research, 192, 106786, https://doi.org/10.1016/j.phrs.2023.106786
[22]. Boopathi, S., Haridevamuthu, B., Mendonca, E., Gandhi, A., Priya, P. S., Alkahtani, S., Al-Johani, N. S., Arokiyaraj, S., Guru, A., Arockiaraj, J., & Malafaia, G., 2023, Combined effects of a high-fat diet and polyethylene microplastic exposure induce impaired lipid metabolism and locomotor behavior in larvae and adult zebrafish. The Science of the Total Environment, 902, 165988, https://doi.org/10.1016/j.scitotenv.2023.165988
[23]. Swanson, D., Block, R., & Mousa, S. A., 2012. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Advances in Nutrition (Bethesda, Md.), 3(1), 1–7, https://doi.org/10.3945/an.111.000893
[24]. Calder, P. C., 2013, Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? British Journal of Clinical Pharmacology, 75(3), 645–662, https://doi.org/10.1111/j.1365-2125.2012.04374.x
[25]. Fazelian, S., Moradi, F., Agah, S., Hoseini, A., Heydari, H., Morvaridzadeh, M., Omidi, A., Pizarro, A. B., Ghafouri, A., & Heshmati, J., 2021, Effect of omega-3 fatty acids supplementation on cardio-metabolic and oxidative stress parameters in patients with chronic kidney disease: A systematic review and meta-analysis. BMC Nephrology, 22(1), 160, https://doi.org/10.1186/s12882-021-02351-9
[26]. Calder, P. C., 2015, Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et Biophysica Acta, 1851(4), 469–484, https://doi.org/10.1016/j.bbalip.2014.08.010
[27]. Wen, J., Satyanarayanan, S. K., Li, A., Yan, L., Zhao, Z., Yuan, Q., Su, K. P., & Su, H., 2024, Unraveling the impact of omega-3 polyunsaturated fatty acids on blood-brain barrier (BBB) integrity and glymphatic function. Brain, Behavior, and Immunity, 115, 335–355, https://doi.org/10.1016/j.bbi.2023.10.018
[28]. Elisia, I., Yeung, M., Kowalski, S., Wong, J., Rafiei, H., Dyer, R. A., Atkar-Khattra, S., Lam, S., & Krystal, G., 2022, Omega 3 supplementation reduces C-reactive protein, prostaglandin E2 and the granulocyte/lymphocyte ratio in heavy smokers: An open-label randomized crossover trial. Frontiers in Nutrition, 9, 1051418, https://doi.org/10.3389/fnut.2022.1051418
[29]. Allam-Ndoul, B., Guénard, F., Barbier, O., & Vohl, M. C., 2017, Effect of different concentrations of omega-3 fatty acids on stimulated THP-1 macrophages. Genes & Nutrition, 12, 7, https://doi.org/10.1186/s12263-017-0554-6
[30]. Flock, M. R., Skulas-Ray, A. C., Harris, W. S., Gaugler, T. L., Fleming, J. A., & Kris-Etherton, P. M, 2014, Effects of supplemental long-chain omega-3 fatty acids and erythrocyte membrane fatty acid content on circulating inflammatory markers in a randomized controlled trial of healthy adults. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 91(4), 161–168, https://doi.org/10.1016/j.plefa.2014.07.006
[31]. Indian Council of Medical Research & National Institute of Nutrition, 2024, Dietary Guidelines for Indians, ICMR-NIN. Available at https://main.icmr.nic.in/sites/default/files/upload_documents/DGI_07th_May_2024_fin.pdf
[32]. Office of Dietary Supplements. (n.d.). Omega-3 fatty acids - Health professional. National Institutes of Health, https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/
[33]. Mason, R. P., & Sherratt, S. C. R., 2017, Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochemical and Biophysical Research Communications, 483(1), 425–429, https://doi.org/10.1016/j.bbrc.2016.12.127
[34]. Asghari, K. M., Saleh, P., Salekzamani, Y., Dolatkhah, N., Aghamohammadzadeh, N., & Hashemian, M., 2024, The effect of curcumin and high-content eicosapentaenoic acid supplementations in type 2 diabetes mellitus patients: A double-blinded randomized clinical trial. Nutrition & Diabetes, 14(1), 14, https://doi.org/10.1038/s41387-024-00274-6
Viewed PDF 272 2 -
 Exploring the Interplay Between Polyphenols, Gut Microbiota, and Polycystic Ovary Syndrome (PCOD): A Comprehensive ReviewAuthor: Monisha PrasadDOI: 10.21522/TIJPH.2013.SE.24.05.Art017
Exploring the Interplay Between Polyphenols, Gut Microbiota, and Polycystic Ovary Syndrome (PCOD): A Comprehensive ReviewAuthor: Monisha PrasadDOI: 10.21522/TIJPH.2013.SE.24.05.Art017Exploring the Interplay Between Polyphenols, Gut Microbiota, and Polycystic Ovary Syndrome (PCOD): A Comprehensive Review
Abstract:
Polycystic Ovary Syndrome (PCOD) is a common endocrine disorder in women of reproductive age, marked by hyperandrogenism, insulin resistance, and chronic anovulation. Emerging research indicates that the gut microbiota significantly contributes to the pathophysiology of PCOD, affecting both metabolic and hormonal balance. Polyphenols, bioactive compounds found in various foods, have shown promise in modulating gut microbiota composition and function, offering potential therapeutic benefits for PCOD. This review examines the intricate interplay between polyphenols, gut microbiota, and PCOD, focusing on how polyphenols may influence PCOD pathogenesis through gut microbiota modulation. We explore the alterations in gut microbiota observed in PCOD patients and how polyphenols may help restore microbial balance, potentially improving metabolic and reproductive health. The review also discusses the underlying molecular mechanisms, such as the regulation of inflammatory pathways, oxidative stress, and insulin signaling, through which polyphenols may exert their effects. By synthesizing findings from preclinical and clinical studies, we highlight the potential of dietary polyphenols as a complementary strategy in PCOD management, while also underscoring the need for more research to determine the most effective dietary interventions and polyphenol formulations. Understanding the relationship between polyphenols and gut microbiota in the context of PCOD offers promising new avenues for developing microbiota-targeted therapies that leverage the beneficial effects of polyphenols to improve outcomes for women with this condition.
Exploring the Interplay Between Polyphenols, Gut Microbiota, and Polycystic Ovary Syndrome (PCOD): A Comprehensive Review
References:
[1]. Singh, S., Pal, N., Shubham, S., Sarma, D. K., Verma, V., Marotta, F., Kumar, M., 2023, Polycystic ovary syndrome: Etiology, current management, and future therapeutics. Journal of Clinical Medicine, 12(4), 1454, https://doi.org/10.3390/jcm12041454
[2]. Tsao, R., 2010, Chemistry and biochemistry of dietary polyphenols. Nutrients, 2(12), 1231–1246, https://doi.org/10.3390/nu2121231
[3]. Shanmugam, G., 2024, Polyphenols: Potent protectors against chronic diseases. Natural Product Research, 1–3, https://doi.org/10.1080/14786419.2024.2386402
[4]. Wu, H. J., & Wu, E., 2012, The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes, 3(1), 4–14, https://doi.org/10.4161/gmic.19320
[5]. Ray, S. K., & Mukherjee, S., 2021, Evolving interplay between dietary polyphenols and gut microbiota—An emerging importance in healthcare. Frontiers in Nutrition, 8, 634944, https://doi.org/10.3389/fnut.2021.634944
[6]. Kasprzak-Drozd, K., Oniszczuk, T., Stasiak, M., & Oniszczuk, A., 2021, Beneficial effects of phenolic compounds on gut microbiota and metabolic syndrome. International Journal of Molecular Sciences, 22(7), 3715, https://doi.org/10.3390/ijms22073715
[7]. Rudrapal, M., Khairnar, S. J., Khan, J., Dukhyil, A. B., Ansari, M. A., Alomary, M. N., Alshabrmi, F. M., Palai, S., Deb, P. K., & Devi, R., 2022, Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials, and mechanism(s) of action. Frontiers in Pharmacology, 13, 806470, https://doi.org/10.3389/fphar.2022.806470
[8]. Yan, Z., Zhong, Y., Duan, Y., Chen, Q., & Li, F., 2020, Antioxidant mechanism of tea polyphenols and its impact on health benefits. Animal Nutrition, 6(2), 115–123, https://doi.org/10.1016/j.aninu.2020.01.001
[9]. Chavez, G. N., Jaworsky, K., & Basu, A., 2023, The effects of plant-derived phytochemical compounds and phytochemical-rich diets on females with polycystic ovarian syndrome: A scoping review of clinical trials. International Journal of Environmental Research and Public Health, 20(15), 6534, https://doi.org/10.3390/ijerph20156534
[10]. Zhou, P., Feng, P., Liao, B., Fu, L., Shan, H., Cao, C., Luo, R., Peng, T., Liu, F., & Li, R., 2024, Role of polyphenols in remodeling the host gut microbiota in polycystic ovary syndrome. Journal of Ovarian Research, 17(1), 69, https://doi.org/10.1186/s13048-024-01354-y
[11]. Shreiner, A. B., Kao, J. Y., & Young, V. B., 2015, The gut microbiome in health and in disease. Current Opinion in Gastroenterology, 31(1), 69–75, https://doi.org/10.1097/MOG.0000000000000139
[12]. Thursby, E., & Juge, N., 2017, Introduction to the human gut microbiota. The Biochemical Journal, 474(11), 1823–1836, https://doi.org/10.1042/BCJ20160510
[13]. Guo, H., Luo, J., & Lin, H., 2023, Exploration of the pathogenesis of polycystic ovary syndrome based on gut microbiota: A review. Medicine, 102(50), e36075, https://doi.org/10.1097/MD.0000000000036075
[14]. Li, Y., Zhu, Y., Li, D., Liu, W., Zhang, Y., Liu, W., Zhang, C., & Tao, T., 2023, Depletion of gut microbiota influents glucose metabolism and hyperandrogenism traits of mice with PCOS induced by letrozole. Frontiers in Endocrinology, 14, 1265152, https://doi.org/10.3389/fendo.2023.1265152
[15]. Wang, X., Qi, Y., & Zheng, H., 2022, Dietary polyphenol, gut microbiota, and health benefits. Antioxidants, 11(6), 1212, https://doi.org/10.3390/antiox11061212
[16]. Cuciniello, R., Di Meo, F., Filosa, S., Crispi, S., & Bergamo, P., 2023, The antioxidant effect of dietary bioactives arises from the interplay between the physiology of the host and the gut microbiota: Involvement of short-chain fatty acids. Antioxidants, 12(5), 1073, https://doi.org/10.3390/antiox12051073
[17]. Ramamurthy, J., 2024, Evaluation of antimicrobial activity of nanoformulated grape seed oil against oral microbes: An in vitro study. World Journal of Dentistry, 15(1), 43–49, https://doi.org/10.5005/jp-journals-10015-2360
[18]. Shabbir, U., Rubab, M., Daliri, E. B., Chelliah, R., Javed, A., & Oh, D. H., 2021, Curcumin, quercetin, catechins and metabolic diseases: The role of gut microbiota. Nutrients, 13(1), 206, https://doi.org/10.3390/nu13010206
[19]. Reygaert, W. C., 2018, Green tea catechins: Their use in treating and preventing infectious diseases. BioMed Research International, 2018, 9105261, https://doi.org/10.1155/2018/9105261
[20]. Fan, F. Y., Sang, L. X., & Jiang, M., 2017, Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules, 22(3), 484, https://doi.org/10.3390/molecules22030484
[21]. Sun, Y., Gao, S., Ye, C., & Zhao, W., 2023, Gut microbiota dysbiosis in polycystic ovary syndrome: Mechanisms of progression and clinical applications. Frontiers in Cellular and Infection Microbiology, 13, 1142041, https://doi.org/10.3389/fcimb.2023.1142041
[22]. Hamamah, S., Iatcu, O. C., & Covasa, M., 2024, Nutrition at the intersection between gut microbiota eubiosis and effective management of type 2 diabetes. Nutrients, 16(2), 269, https://doi.org/10.3390/nu16020269
[23]. Zhang, Y., Yu, W., Zhang, L., Wang, M., & Chang, W., 2022, The interaction of polyphenols and the gut microbiota in neurodegenerative diseases. Nutrients, 14(24), 5373, https://doi.org/10.3390/nu14245373
[24]. Abdelsalam, S. A., Renu, K., Zahra, H. A., Abdallah, B. M., Ali, E. M., Veeraraghavan, V. P., Sivalingam, K., Ronsard, L., Ammar, R. B., Vidya, D. S., Karuppaiya, P., Al-Ramadan, S. Y., & Rajendran, P., 2023, Polyphenols mediate neuroprotection in cerebral ischemic stroke—An update. Nutrients, 15(5), 1107, https://doi.org/10.3390/nu15051107
[25]. Kumar, N., & Goel, N., 2019, Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnology Reports, 24, e00370, https://doi.org/10.1016/j.btre.2019.e00370
[26]. Anantharaju, P. G., Gowda, P. C., Vimalambike, M. G., & Madhunapantula, S. V., 2016, An overview on the role of dietary phenolics for the treatment of cancers. Nutrition Journal, 15(1), 99, https://doi.org/10.1186/s12937-016-0217-2
[27]. He, F. F., & Li, Y. M., 2020, Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. Journal of Ovarian Research, 13(1), 73, https://doi.org/10.1186/s13048-020-00670-3
[28]. Williamson, G., & Sheedy, K., 2020, Effects of polyphenols on insulin resistance. Nutrients, 12(10), 3135, https://doi.org/10.3390/nu12103135
[29]. Zhou, M., Yu, J., Li, X., Ruan, Z., & Yu, S., 2024, Role of the gut microbiota and innate immunity in polycystic ovary syndrome: Current updates and future prospects. Journal of Cellular and Molecular Medicine, 28(8), e18258, https://doi.org/10.1111/jcmm.18258
[30]. Bharali, M. D., Rajendran, R., Goswami, J., Singal, K., & Rajendran, V., 2022, Prevalence of polycystic ovarian syndrome in India: A systematic review and meta-analysis. Cureus, 14(12), e32351, https://doi.org/10.7759/cureus.32351
[31]. Islam, M. T., Mazumder, S., Aniqa, F. T., Uddin, N., Rahman, M. A., Sarkar, C., Mondal, M., Mubarak, M. S., & Rengasamy, K. R. R., 2024, Investigating the hepatoprotective properties of thymol against paracetamol-induced hepatotoxicity. Food Bioscience, 60, 104498, https://doi.org/10.1016/j.fbio.2023.104498
[32]. Azizi, A., Mumin, N. H., & Shafqat, N., 2021, Phytochemicals with anti 5-alpha-reductase activity: A prospective for prostate cancer treatment. F1000 Research, 10, 221, https://doi.org/10.12688/f1000research.51066.3
[33]. Sinan, K. I., Sut, S., Zengin, G., Dall'Acqua, S., Bouyahya, A., Uba, A. I., Ponniya, S. K. M., & Rengasamy, K. R. R., 2024, Integration of chemical characterization, biological activities, and network pharmacology of different extracts from Syzygium rowlandii. Journal of Molecular Structure, 1299, 137117, https://doi.org/10.1016/j.molstruc.2023.137117
[34]. Liu, J., Liu, Y., & Li, X., 2023, Effects of intestinal flora on polycystic ovary syndrome. Frontiers in Endocrinology, 14, 1151723, https://doi.org/10.3389/fendo.2023.1151723
Viewed PDF 197 5 -
 Exploring the Spectrum: Hemoglobinopathies in Pregnancy and their Clinical ImplicationsAuthor: Vinod Kumar NelsonDOI: 10.21522/TIJPH.2013.SE.24.05.Art018
Exploring the Spectrum: Hemoglobinopathies in Pregnancy and their Clinical ImplicationsAuthor: Vinod Kumar NelsonDOI: 10.21522/TIJPH.2013.SE.24.05.Art018Exploring the Spectrum: Hemoglobinopathies in Pregnancy and their Clinical Implications
Abstract:
The present study aimed to investigate the maternal and fetal outcomes of pregnant women presenting to a tertiary healthcare facility with beta thalassemia trait, hemoglobinopathy E, and sickle cell trait. This retrospective study of a series of cases presented to a tertiary healthcare facility, Saveetha Medical College, Thandalam, Chennai between June 2023 and December 2023. The case series included nine pregnant women aged between 21 - 31 years with varying obstetric histories. Most were multigravida with 60.0% having previous caesarean sections. Gestational age at diagnosis ranged from 17 - 35 weeks with one case diagnosed preconception. Mean hemoglobin levels before correction were 8.5 gm/dL, rising to 10.3 gm/dL post-correction. Hematological parameters varied within normal ranges. HPLC revealed heterozygous hemoglobinopathy E, beta thalassemia trait or sickle cell trait in equal proportions. Most deliveries occurred at term, with varying modes of delivery including emergency caesarean sections and vaginal deliveries. Incidence of postpartum hemorrhage and preterm premature rupture of membranes was infrequent, though one case of each occurred. No instances of pre-eclampsia, foetal growth restriction, or maternal or foetal deaths were reported. Neonatal outcomes were generally favorable, with all babies born alive, mostly at term, and satisfactory APGAR scores. Two cases were born with low birth weight. Early and accurate diagnosis facilitated tailored interventions, ultimately lead to positive maternal and neonatal outcomes. These cases serve as poignant reminders of the importance of comprehensive antenatal care, genetic counselling, and specialised management strategies for individuals affected by hemoglobinopathies.
Exploring the Spectrum: Hemoglobinopathies in Pregnancy and their Clinical Implications
References:
[1]. Daigavane, M. M., Jena, R. K., Kar, T. J., 2013. Perinatal outcome in sickle cell anemia: A prospective study from India. Hemoglobin. 37(6), 507-15.
[2]. Modell, B., Darlison, M., 2008. Global epidemiology of hemoglobin disorders and derived service indicators. Bull World Health Organ. 86(6), 480-7.
[3]. Balgir, R. S., 2007. The burden of haemoglobinopathies in India and the challenges ahead. Current Science. 79(11), 1536-47.
[4]. Priyadarsini, B., Mohapatra, K., Naik, M., Behuria, S., 2022. Antenatal screening for hemoglobinopathies with HPLC and their fetomaternal outcome. International Journal of Health Sciences, 6(S9), 2958–2968.
[5]. Colah, R. B., Mukherjee, M. B., Martin, S., Ghosh, K., 2015. Sickle cell disease in tribal populations in India. Indian J Med Res.141(5), 509-15.
[6]. Kohne, E., 2011, Hemoglobinopathies: clinical manifestations, diagnosis, and treatment Dtsch Arztebl Int. 108(31-32), 532-40.
[7]. Mondal, S. K., Mandal, S., 2016. Prevalence of thalassemia and hemoglobinopathy in eastern India: A 10-year high-performance liquid chromatography study of 119,336 cases. Asian J Transfus Sci. 10(1), 105-10.
[8]. Feng, C., Tsoi, W., 1995. A survey of pregnancies that ended in huemoglobin Burt's hydrops foetulis and Cooley's unuemia. The Anthropologist. 6(1), 69-75.
[9]. Balgir, R., Dash, B., Murmu, B., 2004. Blood groups, hemoglobinopathy and G-6-PD deficiency investigations among fifteen major scheduled tribes of Orissa, India. The Anthropologist. 6(1), 69-75.
[10]. Api, O., Breyman, C., Çetiner, M., Demir, C., Ecder, T., 2015. Diagnosis and treatment of iron deficiency anemia during pregnancy and the postpartum period: Iron deficiency anemia working group consensus report. Turk J Obstet Gynecol. 12(3), 173-81.
[11]. Creary, M., Williamson, D., Kulkarni, R., 2007. Sickle cell disease: current activities, public health implications, and future directions. Journal of Women's Health. 16(5), 575-82.
[12]. Jain, B. B., Roy, R. N., Ghosh, S., Ghosh, T., Banerjee, U., Bhattacharya, S. K., 2012. Screening for thalassemia and other hemoglobinopathies in a tertiary care hospital of West Bengal: Implications for population screening. Indian Journal of Public Health. 56(4), 297-300.
[13]. Ghosh, N., Chakrabarti, I., Chakraborty, M., Goswami, B. K., 2013, A community based pilot study on prevalence of hemoglobinopathies among the antenatal women in a rural area of Darjeeling district, West Bengal. International Journal of Medicine and Public Health. 3(2), 69-75.
[14]. Khera, R., Singh, T., Khuana, N., Gupta, N., Dubey, A. P., 2015, HPLC in characterization of hemoglobin profile in thalassemia syndromes and hemoglobinopathies: a clinicohematological correlation. Indian J Hematol Blood Transfus. 31(1), 110-5.
[15]. George, E., Jamal, A. R., Khalid, F., Osman, K. A., 2001, High performance liquid chromatography (HPLC) as a screening tool for classical Beta-thalassaemia trait in malaysia. Malays J Med Sci. 8(2), 40-6.
[16]. Rappaport, V. J., Velazquez, M., Williams, K., 2004, Hemoglobinopathies in pregnancy. Obstet Gynecol Clin North Am. 31(2), 287-317.
[17]. Mensah, C., Sheth, S., 2021, Optimal strategies for carrier screening and prenatal diagnosis of α- and β-thalassemia. Hematology Am Soc Hematol Educ Program. 24(1), 607-13.
[18]. Nisenblat, V., Barak, S., Griness, O. B., Degani, S., Ohel, G., Gonen, R., 2006, Maternal complications associated with multiple cesarean deliveries. Obstet Gynecol. 108(1), 21-6.
[19]. Atrash, H. K., Johnson, K., Adams, M., Cordero, J. F., 2006, Howse J. Preconception care for improving perinatal outcomes: the time to act. Matern Child Health J. 10(5), S3-11.
[20]. Cortés, B. A., 2013, Anemia and transfusion of red blood cells. Colomb Med (Cali). 44(4), 236-42.
[21]. Ghosh, K., Colah, R., Manglani, M., Choudhry, V. P., Verma, I., Madan, N., et al., 2014, Guidelines for screening, diagnosis and management of hemoglobinopathies. Indian J Hum Genet. 20(2), 101-19.
[22]. Ai, S., Cliffe, C., Kidson, G. G., 2021, Antenatal haemoglobinopathy screening - Experiences of a large Australian Centre. Obstet Med. 14(2), 89-94.
[23]. Kladny, B., Williams, A., Gupta, A., Gettig, E. A., Krishnamurti, L., 2011, Genetic counseling following the detection of hemoglobinopathy trait on the newborn screen is well received, improves knowledge, and relieves anxiety. Genetics in Medicine. 13(7), 658-61.
[24]. Chauhan, A., Prasad, M., 2018, Outcome of Pregnancy with Hemoglobinopathy in a Tertiary Care Centre. J Obstet Gynaecol India. 68(5), 394-9.
[25]. Hanprasertpong, T., Kor-anantakul, O., Leetanaporn, R., Suntharasaj, T., Suwanrath, C., Pruksanusak, N., et al., 2013, Pregnancy outcomes amongst thalassemia traits. Arch Gynecol Obstet. 288(5), 1051-4.
[26]. Wellenstein, W. L., Sullivan, S., Darbinian, J., Ritterman, M. L., Greenberg, M., 2019, Adverse pregnancy outcomes in women with sickle cell trait. AJP Rep. 9(4), e346-e52.
[27]. Shanmugam, R., Tharani, M., Abullais, S.S., 2024, Black seed assisted synthesis, characterization, free radical scavenging, antimicrobial and anti-inflammatory activity of iron oxide nanoparticles. BMC Complement Med Ther 24(24), 241. https://doi.org/10.1186/s12906-024-04552-9.
[28]. Habeeb Rahuman H. B., Dhandapani R., Narayanan S., Palanivel V., Paramasivam R., Subbarayalu R., Thangavelu S., Muthupandian S., 2022, Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnol. 16(4), 115-144. Doi: 10.1049/nbt2.12078.
[29]. Wadhwa, R., Paudel, K. R., Chin, L. H., Hon, C. M., Madheswaran, T., Gupta, G., Panneerselvam, J., Lakshmi, T., 2021, Anti-inflammatory and anticancer activities of Naringenin-loaded liquid crystalline nanoparticles in vitro. J Food Biochem. 45(1), e13572. Doi: 10.1111/jfbc.13572.
Viewed PDF 238 9 -
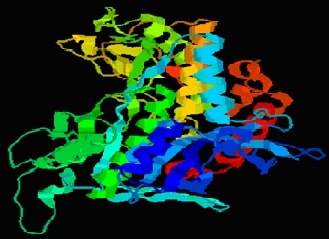 Glutamine Synthase Expression Profiling and Selective Inhibitor for Burkholderia psuedomallei K96243: An In-silico ApproachAuthor: Gopalakrishnan V. KDOI: 10.21522/TIJPH.2013.SE.24.05.Art019
Glutamine Synthase Expression Profiling and Selective Inhibitor for Burkholderia psuedomallei K96243: An In-silico ApproachAuthor: Gopalakrishnan V. KDOI: 10.21522/TIJPH.2013.SE.24.05.Art019Glutamine Synthase Expression Profiling and Selective Inhibitor for Burkholderia psuedomallei K96243: An In-silico Approach
Abstract:
Melioidosis is a potentially fatal infection caused by the Gram-negative bacillus, Burkholderia pseudomallei following an encounter with contaminated soil or surface water. This study was to identify the highly expressed gene in Burkholderia pseudomallei and model the target protein's three-dimensional structure. Further, the study was intended to determine the better binding candidates from the existing drugs against the target protein for melioidosis. The highly expressed gene was identified by KEGG pathway and their target protein was modeled using homology modeling. The modeled structure was validated by PROCHEK and it can be further used for docking studies against existing antibacterial drugs using Argus Lab. The ADME properties of drugs were analyzed by the ADME/Tox WEB. The results revealed that, glutamine synthetase was highly expressed in Burkholderia pseudomallei and its 3D model was generated. The structure validation revealed that, the structure was reliable and reasonable. The docking studies revealed that the Chloramphenicol compound has higher docking score against the protein glutamine synthetase compared to other existing compounds. The acceptable range of the ADME and biological activity prediction of Chloramphenicol compound depicts better pharmacological properties and possible drug-likeliness. This study concluded that, the compound Chloramphenicol may be a better inhibitor for glutamine synthetase protein. This compound may lead to development of effective drug against Melioidosis.
Glutamine Synthase Expression Profiling and Selective Inhibitor for Burkholderia psuedomallei K96243: An In-silico Approach
References:
[2]. Prinsloo, C., Smith, S., Law, M., & Hanson, J., 2023, The Epidemiological, Clinical, and Microbiological Features of Patients with Burkholderia pseudomallei Bacteraemia—Implications for Clinical Management. Tropical Medicine and Infectious Disease, 8(11),481. https://doi.org/10.3390/tropicalmed8110481
[3]. Shaw, T., Assig, K., Tellapragada, C., Wagner, G. E., Choudhary, M., Göhler, A., Eshwara, V. K., Steinmetz, I., Mukhopadhyay, C., 2022, Environmental factors associated with soil revalence of the Melioidosis Pathogen Burkholderia pseudomallei: A Longitudinal Seasonal Study from South West India. Frontiers in Microbiology, 13. https://doi.org/10.3389/fmicb.2022.902996
[4]. Desoutter, A., Deshayes, T., Vorimore, F., Klotoe, B., Durand, B., Colot, J., Wagner-Lichtenegger, G., Steinmetz, I., Tuanyok, A., Laroucau, K., 2024, Isolation of Burkholderia pseudomallei from a goat in New Caledonia: implications for animal and human health monitoring and serological tool comparison. BMC Veterinary Research, 20, 114 https://doi.org/10.1186/s12917-024-03957-5
[5]. Mohapatra, P. R., Mishra, B., 2022, Burden of melioidosis in India and South Asia: Challenges and ways forward. The Lancet Reg Health - Southeast Asia, 2, 100004. https://doi.org/10.1016/j.lansea.2022.03.004
[6]. Wiersinga, W. J., Virk, H. S., Torres, A. G., Currie, B. J., Peacock, S. J., Dance, D. A B., Limmathurotsakul, D., 2018, Melioidosis. Nature Reviews Disease Primers, 4(1), https://doi.org/10.1038/nrdp.2017.107
[7]. Currie, B.J., 2015, Melioidosis: Evolving concepts in epidemiology, pathogenesis, and treatment. In Seminars in respiratory and critical care medicine. Semin Respir Crit Care Med, 36(1):111-25, doi: 10.1055/s-0034-1398389
[8]. Bzdyl, N. M., Moran, C. L., Bendo, J., Sarkar-Tyson, M., 2022, Pathogenicity and virulence of Burkholderia pseudomallei. Virulence, 13(1), 1945-1965, https://doi.org/10.1080/21505594.2022.2139063
[9]. Anggraini, D., Siregar, F. M., Rosdiana, D., Kemal, R. A., Yovi, I., Triani, Z. D., Jasmin, N., Dwijelita, N., Webb, J. R., Mayo, M., Kaestli, M., Currie, B. J. 2024, Epidemiology and genetic diversity of Burkholderia pseudomallei from Riau Province, Indonesia. PLoS Neglected Tropical Diseases, 18(5), e0012195. https://doi.org/10.1371/journal.pntd.0012195
[10]. Gassiep, I., Chatfield, M. D., Permana, B., Burnard, D., Bauer, M. J., Cuddihy, T., Forde, B. M., Mayer-Coverdale, J., Norton, R. E., Harris, P. N. A., 2024, The Genomic Epidemiology of Clinical Burkholderia pseudomallei Isolates in North Queensland, Australia. Pathogens, 13(7), 584 https://doi.org/10.3390/pathogens13070584
[11]. Schully, K. L., Voegtly, L. J., Rice, G. K., Drumm, H., Fitzpatrick, M. C., Malagon, F., Shea, A., Dong, M., Oduro, G., Robberts, F. J. L., Dartey, P. K. A., Owusu-Ofori, A., Clark, D. V., Cer, R. Z., Bishop-Lilly, K. A., 2024, Phylogenetic and phenotypic characterization of Burkholderia pseudomallei isolates from Ghana reveals a novel sequence type and common phenotypes. Frontiers in Microbiology, 15, https://doi.org/10.3389/fmicb.2024.1401259
[12]. Seng, R., Chomkatekaew, C., Tandhavanant, S., Saiprom, N., Phunpang, R., Thaipadungpanit, J., Batty, E. M., Day, N. P., Chantratita, W., West, T. E., Thomson, N. R., Parkhill, J., Chewapreecha, C., Chantratita, N., 2024, Genetic diversity, determinants, and dissemination of Burkholderia pseudomallei lineages implicated in melioidosis in northeast Thailand. Nat Commun., 15, 5699, https://doi.org/10.1101/2023.06.02.543359
[13]. Lim, Y., Vadivelu, J., Mariappan, V., Venkatraman, G., & Vellasamy, K. M. 2023, Effective Therapeutic Options for Melioidosis: Antibiotics versus Phage Therapy. Pathogens, 12(1), 11 https://doi.org/10.3390/pathogens12010011
[14]. Ogata, H., Goto, S., Sato, K., Fujobuchi, W., Bono, H., Kanehisa, M., 1999, KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research, 27, 29-34 DOI: 10.1093/nar/27.1.29
[15]. Grote, A., Hiller. K., Scheer M, et al. 2005, JCat: A novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Research, 33, W526–W531, DOI: 10.1093/nar/gki376
[16]. Schwede, T., Kopp, J., Guex, N., Peitsch, M.C., 2003, SWISS-MODEL: An automated protein homology- modeling server. Nucleic Acids Research, 31, 3381–3385, doi: 10.1093/nar/gkg520
[17]. Sivakumari, K., Rathinabai, A.F.M.C., Kaleenam, P.K., Jayaprakash, P., Srikanth, R., 2010, Molecular docking study of bark-derived components of Cinnamomum cassia on aldose reductase. Indian J Sci Technol, 3, 1081-1088, DOI: 10.17485/ijst/2010/v3i10.8
[18]. Duverna, R., Ablordeppey, S.Y., Lamango, N.S., 2010, Biochemical and Docking Analysis of Substrate Interactions with Polyisoprenylated Methylated Protein Methyl Esterase, Curr Cancer Drug Targets, 10, 634–648 doi: 10.2174/156800910,791859443
[19]. Tamaian, R., Niculescu, V., Anghel, M., 2010, In silico Predictions for Improving permeability properties of Principal Anticancer Anthracyclines through Structural Modification. Bull UASVM Vet Med, 67, 329-336, https://doi.org/10.15835/buasvmcn-vm:67:1:5974
[20]. Muenzner, J., Trébulle, P., Agostini, F., Zauber, H., Messner, C. B., Steger, M., Kilian, C., Lau, K., Barthel, N., Lehmann, A., Textoris-Taube, K., Caudal, E., Egger, A., Amari, F., De Chiara, M., Demichev, V., Gossmann, T. I., Mülleder, M., Liti, G., Ralser, M., 2024, Natural proteome diversity links aneuploidy tolerance to protein turnover. Nature, 630, 149–157, https://doi.org/10.1038/s41586-024-07442-9
[21]. Kumar, U., Manivannan, H., Francis, A.P., Veeraraghavan, V.P., Gayathri, R., Sankaran, S.,2023, Prediction of novel natural small molecules from Schinus molle as an inhibitor of PI3K protein target in cancer cells using in silico screening. Cureus, 15(12), e50863. DOI: 10.7759/cureus.50863
[22]. Narayanaswamy, R., Prabhakaran, V.S., Al-Ansari, M.M., Al-Humaid, L.A., Tiwari, P., 2023. An In Silico Analysis of Synthetic and Natural Compounds as Inhibitors of Nitrous Oxide Reductase (N2OR) and Nitrite Reductase (NIR). Toxics, 11, 660. https://doi.org/10.3390/ toxics11080660
[23]. Zhang, C., Chen, Z., Zhang, M., Jia, S., 2023, KEGG_Extractor: An effective extraction tool for KEGG orthologs. Genes, 14(2), 386. https://doi.org/10.3390/genes14020386
[24]. Wiltgen, M., Algorithms for structure comparison and analysis: Homology Modelling of Proteins, 2019, https://doi.org/10.1016/b978-0-12-809633-8.20484-6
[25]. Stanzione, F., Giangreco, I., Cole, J.C., 2021, Use of molecular docking computational tools in drug discovery. Progress in Medicinal Chemistry, 60, 273–343, https://doi.org/10.1016/bs.pmch.2021.01.004
[26]. Saini, S. R., Binduhayyim, R.I.H., Vishwanath, G., Abdulkhaliq, F., Ali, A., Shashit, S.B., Rajesh, V., Doni, D., Punnoth, P.N., Seyed,A.M., Artak.H., In silico assessment of biocompatibility and toxicity: Molecular docking and dynamics simulation of PMMA-based dental materials for interim prosthetic restorations. J Material Science: Materials in Medicine, 35, 28 https://doi.org/10.1007/s10856-024-06799-7
[27]. En-Nahli, F., Hajji, H., Oua bane, M., 2023, ADMET profiling and molecular docking of pyrazole and pyrazolines derivatives as antimicrobial agents. Arabian J Chemistry, 16(11), 105262, https://doi.org/10.1016/j.arabjc.2023.105262
Viewed PDF 167 1 -
 Adverse Maternal and Fetal Outcomes in Pregnancies Complicated by Diabetes Mellitus: A Case SeriesAuthor: Vinod Kumar NelsonDOI: 10.21522/TIJPH.2013.SE.24.05.Art020
Adverse Maternal and Fetal Outcomes in Pregnancies Complicated by Diabetes Mellitus: A Case SeriesAuthor: Vinod Kumar NelsonDOI: 10.21522/TIJPH.2013.SE.24.05.Art020Adverse Maternal and Fetal Outcomes in Pregnancies Complicated by Diabetes Mellitus: A Case Series
Abstract:
Pregnancy-related diabetes mellitus poses an increased risk of detrimental effects on the health of the mother and fetus. This case series aims to highlight the correlation between poorly managed diabetes and complications such as intrauterine fetal death (IUFD), preterm labor, macrosomia, and neonatal death. We analyzed cases of pregnant women with diabetes mellitus who experienced adverse outcomes within one year at our hospital. The cases were selected based on the seriousness of complications and gestational period at the time of occurrence. Three cases such cases were selected, IUFD was experienced by a 30-year-old woman with a gestational age of 36 weeks;a woman whose age was 29 years also experienced IUFD; whose gestational age was 37 weeks during the occurrence; and another case involved of a mother whose age was 34 and was at 3 months and 4 days of gestation, who underwent preterm delivery leading to neonatal death on the first postoperative day following the surgery. This critical nature of handling diabetes during pregnancy is emphasized in this case series to mitigate the adverse effects and the risks which include, IUFD, premature birth, macrosomia, and neonatal death. Along with continuous monitoring and glycaemic control, to avoid outcomes of complications caused by diabetes mellitus during pregnancy educating the patient is also very important.
Adverse Maternal and Fetal Outcomes in Pregnancies Complicated by Diabetes Mellitus: A Case Series
References:
[1]. Home R., 2024. Gestational Diabetes Mellitus, IDF Diabetes Atlas, 11, 2024.
[2]. Coustan, D. R., 2013. Gestational Diabetes Mellitus, Clin. Chem., 59, 1310–1321.
[3]. McIntyre, H. D., Catalano, P., Zhang, C., Desoye, G., Mathiesen, E. R., and Damm, P., 2019. Gestational diabetes mellitus, Nat. Rev. Dis. Primer, 5, 1–19.
[4]. Hod. M et al., 2015. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care, Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet., 131, 173-211.
[5]. Gupta, Y., Goyal, A., Kalra, S., and Tandon, N., 2020. Variation in the classification of hyperglycaemia in pregnancy and its implication, Lancet Diabetes Endocrinol., 8, 264–266.
[6]. Metzger, B. E., Gabbe, S. G., Persson, B., Buchanan, T. A., Catalano, P. A., Damm, P., Dyer, A. R., Leiva, Ad., Hod, M., Kitzmiler, J. L., Lowe, L. P., McIntyre, H. D., Oats, J. J., Omori, Y., Schmidt, M. I., 2010. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy, Diabetes Care, 33, 676–682.
[7]. Metzger, B. E., Lowe, L. P., Dyer, A. R., Trimble, E. R., Chaovarindr, U., Coustan, D. R., Hadden, D. R., McCance, D. R., Hod, M., McIntyre, H. D., Oats, J. J., Persson, B., Rogers, M. S., Sacks, D. A., 2008. Hyperglycemia and Adverse Pregnancy Outcomes | New England Journal of Medicine. 16,115-144.
[8]. Goyal, A., Gupta, Y., and Tandon, N., 2022. Overt Diabetes in Pregnancy, Diabetes Ther., 13, 589–600.
[9]. Wong, T., Ross, G. P., Jalaludin, B. B., and Flack, J. R., 2013. The clinical significance of overt diabetes in pregnancy, Diabet. Med. J. Br. Diabet. Assoc., 30, 468–474.
[10]. Corrado, F., Pintaudi, B., D’Anna, R., Santamaria, A., Giunta, L., and Di Benedetto, A., 2016. Perinatal outcome in a Caucasian population with gestational diabetes and preexisting diabetes first diagnosed in pregnancy, Diabetes Metab., 42, 122–125.
[11]. Egan, A. M., Dow, M. L., and Vella, A., 2020. A Review of the Pathophysiology and Management of Diabetes in Pregnancy, Mayo Clin. Proc., 95, 2734–2746.
[12]. Metz, T. D., Berry, R. S., Fretts, R. C., Reddy, U. M., and Turrentine, M. A., 2020, Obstetric Care Consensus Management of Stillbirth: (Replaces Practice Bulletin Number 102, March 2009), Am. J. Obstet. Gynecol., 222, B2–B20.
[13]. Maslovich, M. M., and Burke. L. M., 2024. Intrauterine Fetal Demise, in StatPearls, 35, 2091–2098.
[14]. Bekiou, A., and Gourounti. K., 2020. Reduced Fetal Movements and Perinatal Mortality, Mater. Socio-Medica, 32, 227–234.
[15]. Lynch, T. A., Westen, E., Li, D., Katzman, P. J., Malshe, A., and Drennan, K., 2022. Stillbirth in women with diabetes: a retrospective analysis of fetal autopsy reports, J. Matern. Fetal Neonatal Med.,35, 2091–2098.
[16]. Dayal, S., and Hong, P. L., 2024. Premature Rupture of Membranes, in StatPearls, Treasure Island (FL): StatPearls Publishing. Accessed: Aug. 11, 2024.
[17]. Muche, A. A., Olayemi, O. O., and Gete, Y. K., 2020. Effects of gestational diabetes mellitus on risk of adverse maternal outcomes: a prospective cohort study in Northwest Ethiopia, BMC Pregnancy Childbirth, 20, 73.
[18]. Bouvier, D et al., 2019. Risk Factors and Outcomes of Preterm Premature Rupture of Membranes in a Cohort of 6968 Pregnant Women Prospectively Recruited, J. Clin. Med., 8, 1987.
[19]. Yildiz Atar, H., Baatz, J. E., and Ryan, R. M., 2021. Molecular Mechanisms of Maternal Diabetes Effects on Fetal and Neonatal Surfactant, Children, 8, 281.
[20]. Li, Y., Wang, W., and Zhang, D., 2019. Maternal diabetes mellitus and risk of neonatal respiratory distress syndrome: A meta-analysis, Acta Diabetol., 56, 729–740.
[21]. Shanmugam, R., Tharani, M., Abullais, S.S., 2024. Black seed assisted synthesis, characterization, free radical scavenging, antimicrobial and anti-inflammatory activity of iron oxide nanoparticles. BMC Complement Med Ther 24(24), 241.
[22]. Habeeb Rahuman HB., Dhandapani R., Narayanan S., Palanivel V., Paramasivam R., Subbarayalu R., Thangavelu S., Muthupandian S., 2022. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnol. 16,115-144.
[23]. Wadhwa R., Paudel KR., Chin LH., Hon CM., Madheswaran T., Gupta G., Panneerselvam J., Lakshmi T., 2021. Anti-inflammatory and anticancer activities of Naringenin-loaded liquid crystalline nanoparticles in vitro. J Food Biochem. 45, 13572.
Viewed PDF 180 12 -
 Evaluating Antiplatelet Compliance in Recurrent Stroke PatientsAuthor: Samuel MDOI: 10.21522/TIJPH.2013.SE.24.05.Art021
Evaluating Antiplatelet Compliance in Recurrent Stroke PatientsAuthor: Samuel MDOI: 10.21522/TIJPH.2013.SE.24.05.Art021Evaluating Antiplatelet Compliance in Recurrent Stroke Patients
Abstract:
Stroke is a leading cause of death and disability worldwide, with recurrent strokes posing a significant challenge in healthcare. Antiplatelet therapy, including agents such as aspirin and clopidogrel, is fundamental in reducing the risk of recurrent ischemic events. The present study aims to evaluate the current literature on adherence to antiplatelet therapy in recurrent stroke patients and also to identify key factors influencing adherence, explore innovative strategies to improve adherence rates and highlight areas for future research. In conclusion, our study highlights the importance of medication-related factors in the management of recurrent stroke patients. Optimizing antiplatelet therapy regimens, addressing barriers to medication adherence, and mitigating adverse events are crucial steps toward improving secondary stroke prevention strategies and enhancing patient outcomes. However, the effectiveness of these therapies is highly dependent on patient adherence, which remains suboptimal, leading to increased risks of recurrent strokes and other cardiovascular events.
Evaluating Antiplatelet Compliance in Recurrent Stroke Patients
References:
[1]. Kernan, W. N., 2015. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association (vol 45, pg 2160, 2014). Stroke, 46(2), E54-E54. doi:10.1161/STR.0000000000000024.
[2]. Osterberg, L., Blaschke, T., 2005. Adherence to medication. New England journal of medicine, 353(5), 487-497. doi:10.1056/NEJMra050100.
[3]. Algra, A., 2007. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. The Lancet Neurology, 6(2), 115-124. doi:10.1016/S1474-4422(06)70685-8.
[4]. Lowres, N., Giskes, K., Hespe, C., Freedman, B., 2019. Reducing stroke risk in atrial fibrillation: adherence to guidelines has improved, but patient persistence with anticoagulant therapy remains suboptimal. Korean Circulation Journal, 49(10), 883-907. doi:10.4070/kcj.2019.0234.
[5]. Pandya, E. Y., Bajorek, B., 2017. Factors affecting patients’ perception on, and adherence to, anticoagulant therapy: anticipating the role of direct oral anticoagulants. The Patient-Patient-Centered Outcomes Research, 10, 163-185. doi:10.1007/s40271-016-0180-1.
[6]. Ruksakulpiwat, S., Benjasirisan, C., Ding, K., Phianhasin, L., Thorngthip, S., Ajibade, A. D., Voss, J. G., 2023. Utilizing social determinants of health model to understand barriers to medication adherence in patients with ischemic stroke: a systematic review. Patient preference and adherence, 2161-2174. doi:10.2147/PPA.S420059.
[7]. Sabouret, P., Savage, M. P., Fischman, D., Costa, F., 2021. Complexity of antiplatelet therapy in coronary artery disease patients. American Journal of Cardiovascular Drugs, 21(1), 21-34. doi:10.1007/s40256-020-00414-0.
[8]. Diener, H. C., Weimar, C., Weber, R., 2010. Antiplatelet therapy in secondary stroke prevention–state of the art. Journal of Cellular and Molecular Medicine, 14(11), 2552-2560. doi:10.1111/j.1582-4934.2010.01163.x.
[9]. Bushnell, C., McCullough, L. D., Awad, I. A., Chireau, M. V., Fedder, W. N., Furie, K. L., Walters, M. R., 2014. Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 45(5), 1545-1588. doi:10.1161/01.str.0000442009.06663.48.
[10]. De Schryver, E. L. L. M., van Gijn, J., Kappelle, L. J., Koudstaal, P. J., Algra, A., 2005. Non–adherence to aspirin or oral anticoagulants in secondary prevention after ischaemic stroke. Journal of neurology, 252, 1316-1321. doi:10.1007/s00415-005-0858-0.
[11]. Kulkarni, S. P., Alexander, K. P., Lytle, B., Heiss, G., Peterson, E. D.., 2006. Long-term adherence with cardiovascular drug regimens. American heart journal, 151(1), 185-191. doi:10.1016/j.ahj.2005.02.038.
[12]. Passacquale, G., Sharma, P., Perera, D., Ferro, A. 2022. Antiplatelet therapy in cardiovascular disease: Current status and future directions. British Journal of Clinical Pharmacology, 88(6), 2686-2699. doi:10.1111/bcp.15221.
[13]. Hilkens, N. A., Algra, A., Diener, H. C., Bath, P. M., Csiba, L., Hacke, W., Greving, J. P., 2021. Balancing benefits and risks of long-term antiplatelet therapy in noncardioembolic transient ischemic attack or stroke. Stroke. doi:10.1161/STROKEAHA.120.031755.
Viewed PDF 179 2 -
 A Review of Spondias pinnata (L.f.) Kurz's Phytochemical Constituents, Traditional Uses, and Pharmacological Activities: An Important Medicinal Plant in AyurvedaAuthor: Raju BalajiDOI: 10.21522/TIJPH.2013.SE.24.05.Art022
A Review of Spondias pinnata (L.f.) Kurz's Phytochemical Constituents, Traditional Uses, and Pharmacological Activities: An Important Medicinal Plant in AyurvedaAuthor: Raju BalajiDOI: 10.21522/TIJPH.2013.SE.24.05.Art022A Review of Spondias pinnata (L.f.) Kurz's Phytochemical Constituents, Traditional Uses, and Pharmacological Activities: An Important Medicinal Plant in Ayurveda
Abstract:
Spondias pinnata (L.f.) Kurz, commonly known as wild mango or hog plum, is a medicinal tree belonging to the family Anacardiaceae, extensively utilized in traditional systems and codified systems of medicines across the Indian subcontinent and Southeast Asia. Plant parts including roots, bark, leaves, fruits, and seeds are used for medicinal purposes to treat a variety of ailments. Phytochemical analysis has revealed that the presence of various bioactive compounds such as flavonoids, tannins, phenolic acids, saponins, and essential oils, which contribute to its pharmacological activities. Essential oil is rich in monoterpene and sesquiterpene compounds such as α-pinene, caryophyllene, and geraniol. Additionally, other phytoconstituents including β-sitosterol, gallic acid, caffeic acid, and alantolactone have been identified from different parts of the plant. Recent research highlights its antioxidant, anti-inflammatory, antimicrobial, and antidiabetic properties, further validating its traditional uses and suggesting potential for the development of novel therapeutic agents. This review provides a comprehensive overview of the phytochemical properties of S. pinnata, offering insights that may be valuable for future research and the establishment of effective natural drugs.
A Review of Spondias pinnata (L.f.) Kurz's Phytochemical Constituents, Traditional Uses, and Pharmacological Activities: An Important Medicinal Plant in Ayurveda
References:
[1]. Soumyanath, A. 2018, Review of Plants with Anti-Diabetes Mellitus Properties Plants with Anti-Diabetes Mellitus Properties. By Appian Subramoniam. CRC Press . 2016 . 591 pp, $119. ISBN 9781482249897. Journal of natural products.
[2]. S. Swathi, S.S., & K. Lakshman, K.L. 2022, Phytopharmacological and Biological Exertion of Spondias pinnata: (A Review). Oriental Journal Of Chemistry.
[3]. Ndukwe IG, Bello AI, Habila JD, John C. 2007, Phytochemical and antimicrobial screening of the crude petroleum spirit and methanol extracts of the stem bark, leaves and roots of Ficus thoningii (blume). Afr J Biotechnol. 6:2645–2649.
[4]. Oryema C, Ziraba RB, Odyek O, Omagor N, Opio A. 2011, Phytochemical properties and toxicity to brine shrimp of medicinal plants in Erute county, Lira district, Uganda. J Med Plant Res. 5:5450–5457.
[5]. Airy Shaw, H.K.. & Forman, L.L. 1967, The genus Spondias L. (Anacardiaceae) in tropical Asia. Kew Bull. 21: 1 – 19.
[6]. Chandra, D. & Mukherjee, S.K. 2000, Anacardiaceae. In:Singh, N.P., Vohra, J.N., Hajra, P.K. & Singh, D.K. (eds.) Fl. India. Botanical Survey of India, Culcutta. pp. 435 – 510.
[7]. Mabberley's Plant-book A Portable Dictionary of Plants, their Classification and Uses , pp. 474 - 482 DOI: https://doi.org/10.1017/9781316335581 Cambridge University Press 2017
[8]. POWO, 2024, Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet; https://powo.science.kew.org/Retrieved 29 August 2024.
[9]. Bora, N.S, Kakoti B, Gogoi B, Goswami AK. 2014, Ethno-medicinal claims, phytochemistry and pharmacology of Spondias pinnata: A review. International Journal of Pharmaceutical Sciences and Research 5: 1138-1145.
[10]. Salma Sameh, Eman Al-Sayed, Rola M. Labib, Abdel Nasser B. Singab, 2019, Comparative metabolic profiling of essential oils from Spondias pinnata (Linn. F.) Kurz and characterization of their antibacterial activities, Industrial Crops and Products, Volume 137, 468-474.
[11]. Hazra B, Biswas S, Mandal N. 2008, Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med. 8:63.
[12]. Li R, Yang J-J, Song X-Z, Wang Y-F, Corlett RT, Xu Y-K, Hu H-B. 2020, Chemical Composition and the Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities of the Fruit Peel Essential Oil from Spondias pinnata (Anacardiaceae) in Xishuangbanna, Southwest China. Molecules. 25(2):343.
[13]. Santos ÉMD, Ataide JA, Coco JC, Fava ALM, Silvério LAL, Sueiro AC, Silva JRA, Lopes AM, Paiva-Santos AC, Mazzola PG. 2023, Spondias sp: Shedding Light on Its Vast Pharmaceutical Potential. Molecules. 28(4):1862.
[14]. Laksemi, D.A.A.S, 2019, Biological activity of Spondias pinnata: A review. Indonesia Journal of Biomedical Science, 13(2).
[15]. Sai K, Chhetri SBB, Devkota SR, Khatri D. 2021, Evaluation of the Hypoglycemic Potential of Leaves Extract of Spondias pinnata (L.f.) Kurz. from Nepal. Scientific World Journal. 3230351.
[16]. Rao BG.; Raju N J. 2010, Investigation of Hepatoprotective Activity of Spondias pinnata, Int J Pharma Sci Res., 1(3), 193-8.
[17]. Jain, Preeti, Khondker Rufaka Hossain, Tamanna Rashid Mishu, and Hasan Mahmud Reza. 2013, Antioxidant and Antibacterial Activities of Spondias pinnata Kurz. Leaves. European Journal of Medicinal Plants, 4 (2):183-95.
[18]. Ghate NB, Hazra B, Sarkar R, Mandal N. 2014, In vitro anticancer activity of Spondias pinnata bark on human lung and breast carcinoma. Cytotechnology. 66(2):209-18.
[19]. Mondal, S., Bhar, K., Panigrahi, N., Mondal, P., Nayak, S., Barik, R. P., & Aravind, K. 2021, A Tangy Twist Review on Hog-Plum: Spondias pinnata (L.f.) Kurz. Journal of Natural Remedies, 21(1), 1–25.
[20]. Chaudhuri D, Ghate NB, Singh SS, Mandal N. 20155, Methyl gallate isolated from Spondias pinnata exhibits anticancer activity against human glioblastoma by induction of apoptosis and sustained extracellular signal-regulated kinase 1/2 activation. Pharmacogn Mag. 11(42):269-76.
[21]. Patathananone S, Daduang J, Koraneekij A, Li C. 2019, Tyrosinase Inhibitory Effect, Antioxidant and Anticancer Activities of Bioactive Compounds in Ripe Hog Plum (Spondias Pinnata) Fruit Extracts. Orient J Chem 35(3).
Viewed PDF 288 14 -
 Histone Demethylase (KDM3A) Regulation and Its Impact on Estrogen Receptor-Positive Breast Cancer ProgressionAuthor: Periasamy AnbuDOI: 10.21522/TIJPH.2013.SE.24.05.Art023
Histone Demethylase (KDM3A) Regulation and Its Impact on Estrogen Receptor-Positive Breast Cancer ProgressionAuthor: Periasamy AnbuDOI: 10.21522/TIJPH.2013.SE.24.05.Art023Histone Demethylase (KDM3A) Regulation and Its Impact on Estrogen Receptor-Positive Breast Cancer Progression
Abstract:
The histone demethylase-encoding gene KDM3A is a crucial modulator of estrogen receptor (ER) signaling, impacting the development of ER-positive breast cancer. Resistance develops despite the effectiveness of endocrine drugs that target ER signaling, necessitating a deeper understanding of the underlying molecular processes. This work investigated KDM3A depletion in ER-positive breast cancer cells to gain a better understanding of how it impacts estrogen-induced gene expression and its potential as a therapeutic target in endocrine-resistant breast cancer. We used the GEO dataset GSE68918, which contains gene expression profiles from MCF-7 cell lines treated with KDM3A RNA (siKDM3A) and scrambled RNA (siSCR), to find 845 differentially expressed genes (DEGs). Following KDM3A knockdown, 402 of these genes showed upregulation, and 444 showed downregulation. Significant downregulation was observed for BRCA1 and CCNB1, but a notable upregulation was observed for CEACAM6. This suggests that KDM3A is involved in signaling through estrogen receptors, repairing DNA, and regulating the cell cycle. A study of protein-protein interaction (PPI) networks showed that hub genes, including TOP2A, CCNA2, and CCNB1, are essential for KDM3A-related networks. Significant enrichment in the p53 signaling pathway, DNA replication, and cell division was found by Gene Ontology (GO) and KEGG pathway studies, highlighting the influence of KDM3A on important biological processes. These findings suggest that KDM3A could be a valuable therapeutic target in the management of breast cancer endocrine resistance. Future research should look at the therapeutic potential of KDM3A inhibitors to enhance the efficacy of treatment for endocrine-resistant breast cancer, particularly when combined with other forms of therapy.
Histone Demethylase (KDM3A) Regulation and Its Impact on Estrogen Receptor-Positive Breast Cancer Progression
References:
[1]. Early Breast Cancer Trialists Collaborative Group., 1998, Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet, 351, 1451–1467.
[2]. Miller, D.W., 2012, Endocrine Therapy for Breast Cancer: Past, Present, and Future. Hormone Research in Paediatrics, 78, 294–302.
[3]. Johnston, S.R., 2010, Endocrine-Resistant Breast Cancer: New Treatment Strategies. Clinical Cancer Res, 16, 1979–1987.
[4]. Vinitha, P., 2024, Utilizing thiol-protected bimetal nanoclusters to enhance the therapeutic potential of seaweed bioactive compounds in oral cancer treatment. Oral Oncol Rep, 10, 100552.
[5]. Sotiriou, C., Wirapati, P., Loi, S., Harris, A., Fox, S., Smeds, J., et al., 2006, Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst, 298, 262–272.
[6]. Carey, L.A., Perou, C.M., Livasy, C.A., Dressler, L.G., Cowan, D., Conway, K., et al., 2006, Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA, 295, 2492–2502.
[7]. Huber, W., von Heydebreck, A., Sültmann, H., Poustka, A., Vingron, M., 2002, Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics, 18, S96–S104.
[8]. Geetha, S., 2024, Combining radiation therapy with microbubbles: An emerging and promising approach to cancer treatment. Oral Oncol Rep, 10, 100493.
[9]. Wade, M.A., Jones, D., Wilson, L., Stockley, J., Coffey K, Robson CN, et al., 2015, The histone demethylase enzyme KDM3A is a key estrogen receptor regulator in breast cancer. Nucleic Acid Res, 43, 196–207.
[10]. Gaughan, L., 2013, KDM4B is a master regulator of the estrogen receptor signaling cascade. Nucleic Acid Res, 41, 6892–6904.
[11]. Luo, S., 2010, The emerging functions of histone demethylases in cancer. Nature Rev Cancer, 10, 305–318.
[12]. Islam, S., Amin, M.A., Rengasamy, K.R.R., Mohiuddin, A.K.M., Mahmud, S., 2024, Structure-based pharmacophore modeling for precision inhibition of mutant ESR2 in breast cancer: A systematic computational approach. Cancer Med, 13, e70074.
[13]. Nautiyal, M., Ganapathy, D., Ameya, K.P., Sekar, D., 2024, Analysis of Carboplatin and STAT3 in the Breast Cancer MCF7 Cell Line. Texila Int J Public Health, 12, 1-7.
[14]. Smyth, G.K., 2005, Limma: Linear Models for Microarray Data. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer-Verlag; pp. 397–420.
[15]. Dunning, M.J., 2014, db: Illumina HumanHT12v4 annotation data (chip illuminaHumanv4). R package version, 1.
[16]. Szklarczyk, D., Gable, A.L., Lyon, D., Junge, A., Wyder, S., Huerta-Cepas, J., et al., 2019, STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acid Res, 47, D607–D613.
[17]. Shannon, P., Markiel, A., Ozier, O., Baliga, N.S., Wang, J.T., Ramage, D., et al., 2003, Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res, 13, 2498–2504.
[18]. Ashburner, M., Ball, C.A., Blake, J.A., Botstein, D., Butler, H., Cherry, J.M., et al., 2000, Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet, 25, 25–29.
[19]. Kanehisa, M., Goto, S., 2000, KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acid Res, 28, 27–30.
[20]. Kanehisa, M., Araki, M., Goto, S., Hattori, M., Hirakawa, M., Itoh, M., et al., 2008, KEGG for linking genomes to life and the environment. Nucleic Acid Res, 36: D480-4.
[21]. Teichmann, S.A., Babu, M.M., 2004, Gene regulatory network growth by duplication. Nat Genet, 36, 492–496.
[22]. Ahmad, Y., Lamond, A.I., 2014, A perspective on protein complex mapping. Nat Rev Mol Cell Biol, 15, 701–714.
[23]. Duxbury, M.S., Ito, H., Benoit, E., Ashley, S.W., Whang, E.E., 2004, CEACAM6 is a determinant of pancreatic adenocarcinoma cellular invasiveness. Br J Cancer, 91, 1384–1390.
[24]. Dannenberg, J.H., David, G., Zhong, S., van der Torre, J., Wong, W.H., Depinho, R.A., 2005, mSin3A corepressor regulates diverse transcriptional networks governing normal and neoplastic growth and survival. Genes Dev, 19, 1581–1595.
[25]. Nevanlinna, H., Bartek, J., 2006, The CHEK2 gene and inherited breast cancer susceptibility. Oncogene, 25, 5912–5919.
[26]. Iwase, S., Lan, F., Bayliss, P., de la Torre-Ubieta, L., Huarte, M., Qi, H.H., et al., 2007, The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell, 128, 1077–1088.
[27]. Moulder, S.L., Borges, V.F., Baetz, T., Mcspadden, T., Haney, P., Gao, D., 2018, Phase I study of KDM3A inhibitor GSK-J1 in patients with ER-positive breast cancer. Clin Cancer Res, 24, 5598–5605.
[28]. Grossman, R.L., Heath, A.P., Ferretti, V., Varmus, H.E., Lowy, D.R., Kibbe, W.A., et al., 2016, Toward a shared vision for cancer genomic data. N Engl J Med, 375, 1109–1112.
Viewed PDF 165 4 -
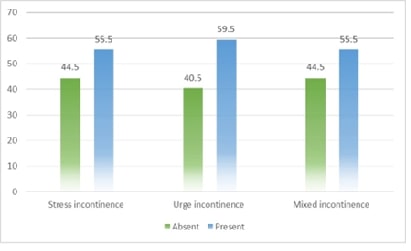 Prevalence and Predictors of Urinary Incontinence in South Indian Women: A Hospital-Based Analytical Cross-Sectional StudyAuthor: Geetha BirudalaDOI: 10.21522/TIJPH.2013.SE.24.05.Art024
Prevalence and Predictors of Urinary Incontinence in South Indian Women: A Hospital-Based Analytical Cross-Sectional StudyAuthor: Geetha BirudalaDOI: 10.21522/TIJPH.2013.SE.24.05.Art024Prevalence and Predictors of Urinary Incontinence in South Indian Women: A Hospital-Based Analytical Cross-Sectional Study
Abstract:
The present study assesses the prevalence of urinary incontinence and evaluates the risk elements related to QUID-derived urge and stress incontinence scores. This was a cross-sectional analysis of women attending the outpatient department and inpatient ward section of Obstetrics and Gynaecology at Saveetha Institute of Medical College Thandalam Chennai. This study included 299 women; the mean (SD) age was 54.4 years (7.7). The proportion of women with Normal and LSCS were 59.5% and 40.5%, respectively. The proportion of women drinking coffee was 74.2%, with recurrent urinary tract infection was 40.1%, with diabetes was 32,4%, with obesity was 37.8%, with a history of constipation was 70.2%, and history of pelvic surgery was 23.4%. The mean (SD) urge score among women with a normal vaginal delivery was 8.19 (2.78) and among those with LSCS was 4.57 (1.99); the difference (higher scores among women with NVD, in comparison with LSCS) was found to be statistically significant. The mean (SD) stress score among women with a normal vaginal delivery was 7.52 (2.78) and among those with LSCS was 4.26 (2.14); the difference (higher scores among women with NVD, in comparison with LSCS) was found to be statistically significant. Also, the results showed that women with coffee drinking, diabetes, hypertension, obesity, constipation, and without pelvic surgeries had significantly higher stress incontinence scores in comparison to their counterparts. The mean (SD) stress scores showed an increasing trend with age – the difference in stress scores by age groups was found to be a statistic.
Prevalence and Predictors of Urinary Incontinence in South Indian Women: A Hospital-Based Analytical Cross-Sectional Study
References:
[1]. Singh, U., Agarwal, P., Verma, M. L., Dalela, D., Singh, N., & Shankhwar, P. 2013, Prevalence and risk factors of urinary incontinence in Indian women: A hospital-based survey. Indian Journal of Urology, 29(1), 31–36.
[2]. Peyrat, L., Haillot, O., Bruyere, F., Boutin, J. M., Bertrand, P., & Lanson, Y. 2002, Prevalence and risk factors of urinary incontinence in young and middle-aged women. BJU International, 89(1), 61–66.
[3]. Tsakiris, P., Oelke, M., & Michel, M. C. 2008, Drug-Induced Urinary Incontinence. Drugs & Aging, 25(7), 541–549. https://doi.org/10.2165/00002512-200825070-00001.
[4]. Sakondhavat, C., Choosuwan, C., Kaewrudee, S., Soontrapa, S., & Louanka, K. 2007, Prevalence and risk factors of urinary incontinence in Khon Kaen menopausal women. Journal of the Medical Association of Thailand Chotmaihet Thangphaet, 90(12), 2553–2558.
[5]. Danforth, K. N., Townsend, M. K., Lifford, K., Curhan, G. C., Resnick, N. M., & Grodstein, F. 2006, Risk Factors for Urinary Incontinence among Middle-aged Women. American Journal of Obstetrics and Gynecology, 194(2), 339–345. https://doi.org/10.1016/j.ajog.2005.07.051.
[6]. Ajith, A. K., Rekha, A., Duttagupta, S., Murali, V., Ramakrishnan, D., & Krishnapillai, V. 2019, Prevalence and Factors of Urinary Incontinence among Postmenopausal Women Attending the Obstetrics and Gynecology Outpatient Service in a Tertiary Health Care Center in Kochi, Kerala. Indian Journal of Community Medicine: Official Publication of the Indian Association for the Prevention of Social Medicine, 44(Suppl 1), S30–S33.
[7]. Markland, A. D., Richter, H. E., Fwu, C.-W., Eggers, P., & Kusek, J. W. 2011, Prevalence and Trends of Urinary Incontinence in Adults in the United States, 2001 to 2008. The Journal of Urology, 186(2), 589–593. https://doi.org/10.1016/j.juro.2011.03.114.
[8]. Huang, K. 2016, Health Care-Seeking Behaviors among Women Suffering from Urinary Incontinence. Journal of Yoga & Physical Therapy, 6. [invalid URL removed].
[9]. Brandt, F., Solomayer, E.-F., & Sklavounos, P. 2021, Psychometric properties of the German-language questionnaire for urinary incontinence diagnosis (QUID) in women with urinary incontinence. Archives of Gynecology and Obstetrics, 304(5), 1233–1242. https://doi.org/10.1007/s00404-021-06167-8.
[10]. Bradley, C. S., et al. 2010, The Questionnaire for Urinary Incontinence Diagnosis (QUID): Validity and Responsiveness.
[11]. Irwin, D. E., Abrams, P., Cartwright, R., deLancey, J. O., Kopp, Z. S., & Patrick, D. L. 2011, Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU International, 101(3), 1202–1210. https://doi.org/10.1111/j.1464-410X.2010.09993.x.
[12]. Townsend, M. K., Gousse, A. V., Laughlin, G. A., Wasserman, M., Liu, H., & Aragia, M. 2007, Incidence and Remission of Urinary Incontinence in Middle-aged Women. American Journal of Obstetrics and Gynecology, 197(2), 167.e1–167.e5.
[13]. Hannestad, Y. S., Rortveit, G., Daltveit, A. K., & Hunskaar, S. 2003, Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG: An International Journal of Obstetrics and Gynaecology, 110(3), 247–254.
[14]. Liu, B., Wang, L., Huang, S. S., Wu, Q., & Wu, D. L. 2014, Prevalence and risk factors of urinary incontinence among Chinese women in Shanghai. International Journal of Clinical and Experimental Medicine, 7(3), 686–696.
[15]. Abrams, P., Cardozo, L., Fall, M., Griffiths, D., Kontsevaya, L., & Moore, D. 2003, The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology, 61(1), 37–49.
[16]. Diokno, A. C., Burgio, K. L., Arnold, E. P., Hunskaar, S., Mallett, V. T., & Herzog, A. R. 2000, Epidemiology and Natural History of Urinary Incontinence. Reviews in Urology [Online]. https://doi.org/10.1007/s001920070021.
[17]. Coyne, K. S., Kvasz, M., Ireland, A. M., Milsom, I., Kopp, Z. S., & Chapple, C. R. 2012, Urinary Incontinence and its Relationship to Mental Health and Health-Related Quality of Life in Men and Women in Sweden,1 the United Kingdom, and the United States. European Urology, 61(1), 88–95. 2 https://doi.org/10.1016/j.eururo.2011.07.049.
[18]. Coyne, K. S., et al. 2009, The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU International, 103(Suppl 3), 4–11.
[19]. Handa, V. L., Blomquist, J. L., McDermott, K. C., Friedman, S., & Muñoz, A. 2012, Pelvic floor disorders after vaginal birth: effect of episiotomy, perineal laceration, and operative birth. Obstetrics and Gynecology, 119(2 Pt 1), 233–239. https://doi.org/10.1097/AOG.0b013e318240df4f.
[20]. Sultan, A. H., Kamm, M. A., Hudson, C. N., Thomas, J. M., & Bartram, C. I. 1993, Anal-Sphincter Disruption during Vaginal Delivery. New England Journal of Medicine, 329(26), 1905–1911.
[21]. Wu, J. M., et al. 2014, Prevalence and Trends of Symptomatic Pelvic Floor Disorders in U.S. Women. Obstetrics and Gynecology, 123(1), 141–148.
[22]. Gleason, J. L., Richter, H. E., Redden, D. T., Goode, P. S., Burgio, K. L., & Markland, A. D. 2013, Caffeine and urinary incontinence in US women. International Urogynecology Journal, 24(2), 295–302.
[23]. Brown, J. S., Vittinghoff, E., Lin, F., Nyberg, L. M., Kusek, J. W., & Kanaya, A. M. 2006, Prevalence and Risk Factors for Urinary Incontinence in Women With Type 2 Diabetes and Impaired Fasting Glucose. Diabetes Care, 29(6), 1307–1312.
[24]. Jackson, S. L., Scholes, D., Boyko, E. J., Abraham, L., & Fihn, S. D. 2006, Predictors of urinary incontinence in a prospective cohort of postmenopausal women. Obstetrics and Gynecology, 108(4), 855–862.
[25]. Haylen, B. T., et al. 2010, An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. International Urogynecology Journal, 21(1), 5–26. https://doi.org/10.1007/s00192-009-0976-9.
[26]. Rogers, R. G., Ninivaggio, C., Gallagher, K., Borders, A. N., Qualls, C., & Leeman, L. M. 2017, Pelvic floor symptoms and quality of life changes during first pregnancy: a prospective cohort study. International Urogynecology Journal, 28(11), 1701–1707.
[27]. Minassian, V. A., Stewart, W. F., & Wood, G. C. 2008, Urinary incontinence in women: variation in prevalence estimates and risk factors. Obstetrics and Gynecology, 111(2 Pt 1), 324–331.
[28]. Hannestad, Y. S., Rortveit, G., Sandvik, H., Hunskaar, S., & Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag 2000, A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. Journal of Clinical Epidemiology, 53(11), 1150–1157. https://doi.org/10.1016/s0895-4356(00)00232-8.
[29]. Nygaard, I., et al. 2008, Prevalence of symptomatic pelvic floor disorders in US women. JAMA, 300(11), 1311–1316. https://doi.org/10.1001/jama.300.11.1311.
[30]. Lukacz, E. S., Lawrence, J. M., Contreras, R., Nager, C. W., & Luber, K. M. 2006, Parity, mode of delivery, and pelvic floor disorders. Obstetrics and Gynecology, 107(6), 1253–1260. https://doi.org/10.1097/01.AOG.0000218096.54169.34.
Viewed PDF 221 4 -
 Hydrothermal Method of Synthesis of β-Ag2MoO4 Nanorods as a Peroxidase Mimetic for Glucose SensingAuthor: Vinitha PackirisamyDOI: 10.21522/TIJPH.2013.SE.24.05.Art025
Hydrothermal Method of Synthesis of β-Ag2MoO4 Nanorods as a Peroxidase Mimetic for Glucose SensingAuthor: Vinitha PackirisamyDOI: 10.21522/TIJPH.2013.SE.24.05.Art025Hydrothermal Method of Synthesis of β-Ag2MoO4 Nanorods as a Peroxidase Mimetic for Glucose Sensing
Abstract:
In this study, β-Ag₂MoO₄ nanorods were synthesized using a hydrothermal method, aiming to explore their potential as a sensing material for L-glucose detection. The synthesis process was optimized to produce uniform nanorods with high crystallinity and surface area, which is essential for enhancing sensor performance. The synthesized β-Ag2MoO4 nanorods characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM), confirmed the successful formation of β-Ag₂MoO₄ nanorods with desired morphological features. The vibrations existing in β-Ag2MoO4 nanorods were characterized by FT-IR and Raman spectroscopic studies. The electrochemical properties of the nanorods were evaluated, demonstrating their sensitivity and selectivity towards L-glucose. The nanorods exhibited a rapid response time, low detection limit, and excellent reproducibility, making them promising candidates for glucose sensors. This work underscores the potential of hydrothermally synthesized β-Ag₂MoO₄ nanorods in the development of advanced glucose sensing platforms, offering a new approach for the efficient and reliable monitoring of glucose levels in medical diagnostics.
Hydrothermal Method of Synthesis of β-Ag2MoO4 Nanorods as a Peroxidase Mimetic for Glucose Sensing
References:
[1]. Shahsavari, M., Mortazavi, M., Tajik, S., Sheikhshoaie, I., & Beitollahi, H. 2022, Synthesis and Characterization of GO/ZIF-67 Nanocomposite: Investigation of Catalytic Activity for the Determination of Epinine in the Presence of Dobutamine. Micromachines, 13(1), 88.
[2]. Bharathi, D., Wadaan, M. A., Mythili, R., & Lee, J. 2024, Synthesis of chitosan and gum arabic functionalized silver nanocomposite for efficient removal of methylene blue and antibacterial activity. Polymers for Advanced Technologies, 35(4), e6356.
[3]. Arularasu, M. V. 2024, Biosynthesis, structural characterization and humidity sensing properties of cellulose/ZnO nanocomposite. Sensing and Bio-Sensing Research, 45, 100673.
[4]. Pushpalatha, S., Arularasu, M. V., Palanivel, C., Rajendran, T. V., & Manikandan, A. 2024, Green synthesis of cellulose/ZrO2 nanocomposite: assessment of antibacterial and photocatalytic activity. Biomass Conversion and Biorefinery, 1–8.
[5]. Tang, X., Li, H., Zhang, T., Zhong, J., & Du, H. 2022, P123-assisted hydrothermal synthesis of Ag2MoO4 with enhanced photocatalytic performance. Inorganic Chemistry Communications, 141, 109613.
[6]. Appiagyei, A. B., Asiedua-Ahenkorah, L., Bathula, C., Kim, H. S., Han, S. S., Rao, K. M., & Anang, D. A. 2023, Rational design of sucrose-derived graphitic carbon coated MnMoO4 for high performance asymmetric supercapacitor. Journal of Energy Storage, 58, 106383.
[7]. Tourchi Moghadam, M. T., Seifi, M., Jamali, F., Azizi, S., & Askari, M. B. 2022, ZnWO4-CNT as a superior electrode material for ultra-high capacitance supercapacitor. Surfaces and Interfaces, 32, 102134.
[8]. Sousa, G. da S., Nobre, F. X., Araújo Júnior, E. A., Sambrano, J. R., Albuquerque, A. dos R., Bindá, R. dos S., … de Matos, J. M. E. 2020, Hydrothermal synthesis, structural characterization and photocatalytic properties of β-Ag2MoO4 microcrystals: Correlation between experimental and theoretical data. Arabian Journal of Chemistry, 13(1), 2806–2825.
[9]. Mispa, K. J., Subramaniam, P., & Murugesan, R. 2014, Studies on polyaniline/silver molybdate nanocomposites. International Journal of Nanoscience, 13(1), 1050002.
[10]. Safartoobi, A., Mazloom, J., & Ghodsi, F. E. 2023, Silver/molybdenum metal-organic framework derived Ag2MoO4 nanoparticles as novel electrode for high-performance supercapacitor. Journal of Energy Storage, 68, 107818.
[11]. Jayanthi, P., Duraimurugan, J., Prabhu, S., Siranjeevi, R., Mary Anjalin, F., and Bhuvaneswari, N. 2024, Enhanced electrochemical performance of CoMoO4/r-GO composites as electrode material for supercapacitor application. Diamond and Related Materials, 148, 111361.
[12]. Maity, S., Bain, D., Chakraborty, S., Kolay, S., & Patra, A. 2020, Copper Nanocluster (Cu23NC)-Based Biomimetic System with Peroxidase Activity. ACS Sustainable Chemistry and Engineering, 8(49), 18335-18344.
[13]. Wang, N., Han, Z., Fan, H., & Ai, S. 2015, Copper nanoparticles modified graphitic carbon nitride nanosheets as a peroxidase mimetic for glucose detection. RSC Advances, 5(111), 91302–91307.
[14]. Fabbro, M. T., Saliby, C., Rios, L. R., La Porta, F. A., Gracia, L., Li, M. S., … Longo, E. 2015, Identifying and rationalizing the morphological, structural, and optical properties of β-Ag2MoO4 microcrystals, and the formation process of Ag nanoparticles on their surfaces: Combining experimental data and first-principles calculations. Science and Technology of Advanced Materials, 16(6).
[15]. Malathi, A., Priyadharsan, A., Handayani, M., Hasan, I., Divya, G., Sivaranjani, K., & Sivakumar, S. 2024, Boosted solar-driven photocatalysis: silver molybdate/reduced graphene oxide nanocomposites for methylene blue decomposition. Ionics, 30(3), 1603-1614.
[16]. Senthil, R. A., Wu, Y., Liu, X., & Pan, J. 2021, A facile synthesis of nano AgBr attached potato-like Ag2MoO4 composite as highly visible-light active photocatalyst for purification of industrial waste-water. Environmental Pollution, 269, 116034.
[17]. Syed, A., AL-Shwaiman, H. A., Al Khulaifi, M. M., Al Zahrani, R. R., Almajhdi, F. N., & Elgorban, A. M. 2021, Integrating plasmonic effect and nano-heterojunction formation for boosted light harvesting and photocatalytic performance using CaWO4/Ag2MoO4 and its antibacterial applications. Materials Science in Semiconductor Processing, 133, 105921.
[18]. Balasurya, S., Okla, M. K., AL-ghamdi, A. A., Al-amri, S. A., Alatar, A. A., Abdel-Maksoud, M. A., … Khan, S. S. 2023, CaMoO4 modified nanorods-branched Ag2MoO4 a nano-diatomic heterojunction as efficient visible-light-driven photocatalysts for water remediation processes and antimicrobial applications. Materials Today Communications, 34, 104945.
[19]. Lopes, F. H. P., Noleto, L. F. G., Vieira, V. E. M., de Sousa, P. B., Jucá, A. C. S., Oliveira, Y. L., … Cavalcante, L. S. 2023, Experimental and Theoretical Correlation of Modulated Architectures of β-Ag2MoO4 Microcrystals: Effect of Different Synthesis Routes on the Morphology, Optical, Colorimetric, and Photocatalytic Properties. Journal of Inorganic and Organometallic Polymers and Materials, 33(2), 424–450.
[20]. Nandi, I., Chall, S., Chowdhury, S., Mitra, T., Roy, S. S., & Chattopadhyay, K. 2018, Protein Fibril-Templated Biomimetic Synthesis of Highly Fluorescent Gold Nanoclusters and Their Applications in Cysteine Sensing. ACS Omega, 3(7), 7703–7714.
[21]. Saylan, Y., Erdem, Ö., Inci, F., & Denizli, A. 2020, Advances in biomimetic systems for molecular recognition and biosensing. Biomimetics. 5(2), 20.
Viewed PDF 177 6 -
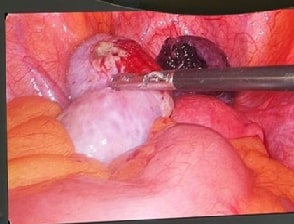 A Rare Case of Torsion of Hydrosalpinx Masquerading as Torsion of the OvaryAuthor: Deepthi PDOI: 10.21522/TIJPH.2013.SE.24.05.Art026
A Rare Case of Torsion of Hydrosalpinx Masquerading as Torsion of the OvaryAuthor: Deepthi PDOI: 10.21522/TIJPH.2013.SE.24.05.Art026A Rare Case of Torsion of Hydrosalpinx Masquerading as Torsion of the Ovary
Abstract:
Isolated fallopian tube torsion is an infrequent but significant cause of acute pelvic pain, often difficult to diagnose pre-operatively due to its symptom overlap with other conditions such as ovarian cyst torsion and appendicitis. Hydrosalpinx, a condition typically asymptomatic, can present with acute pain when complicated by torsion. This case report highlights the diagnostic challenges and management strategies associated with hydrosalpinx torsion. A 35-year-old woman with a history of Polycystic Ovarian Disease (PCOD) and previous sterilization presented with acute right lower abdominal pain and vomiting. Initial ultrasound suggested a right ovarian hemorrhagic cyst, leading to a preoperative diagnosis of cyst torsion. However, diagnostic laparoscopy revealed a 6x4 cm gangrenous hydrosalpinx on the left with four complete twists, and significant congestion in the right fallopian tube, while the right ovary appeared normal. Surgical intervention included a left salpingo-oophorectomy and right partial salpingectomy. Histopathological examination confirmed the presence of a dermoid cyst in the left ovary and extensive congestion in the fallopian tube. Isolated fallopian tube torsion is exceedingly rare, with an estimated incidence of 1 in 1.5 million. This case emphasizes the importance of including hydrosalpinx torsion in differential diagnoses, particularly when clinical symptoms are inconsistent with radiological findings. Accurate and timely diagnosis is critical for appropriate management and to prevent potential complications.
A Rare Case of Torsion of Hydrosalpinx Masquerading as Torsion of the Ovary
References:
[1]. Varghese, S., Seldon, Y., Raperport, C., Rinne, N., Patel, K., Zaid, R. Z., 2024, Isolated fallopian tube torsion: A systematic review of case reports. European Journal of Obstetrics and Gynecology and Reproductive Biology. 296: 140–147. doi:10.1016/j.ejogrb.2024.02.050
[2]. Schwartz, B., Weerasooriya, N., Mercier, R., Gould, S., Saul, D., Berman, L., 2024, Factors Associated With Isolated Fallopian Tube Torsion in Pediatric Patients. Journal of Pediatric Surgery;59: 1538–1544. doi:10.1016/j.jpedsurg.2024.03.054
[3]. Yazawa, R., Yazawa, H., Anjyo, K., Inazuki, A., 2024, Four cases of isolated fallopian tube torsion successfully treated with laparoscopic surgery: A case series. Fukushima Journal of Medical Science; 70: 163–168. doi:10.5387/fms.23-00021
[4]. De Leon Palabrica, P. B., Mallen, M. T. B., Tan, G. A. C., 2023, Isolated fallopian tube torsion in an early adolescent. Philippine Journal of Obstetrics and Gynecology; 47: 325. doi:10.4103/pjog.pjog_69_23
[5]. Narayanan, S., Bandarkar, A., Bulas, D. I., 2014, Fallopian tube torsion in the pediatric age group: radiologic evaluation. J Ultrasound Med. 33: 1697–1704. doi:10.7863/ultra.33.9.1697
[6]. Dixit, K., Setty, J. P., Jassawalla, N., Srikanth., 2024, Journal of Postgraduate Gynecology & Obstetrics: Torsion Of A Hydrosalpinx – a Diagnostic Puzzle. Available: https://www.jpgo.org/2016/02/torsion-of-hydrosalpinx-diagnostic.html
[7]. Antoniou, N., Varras, M., Akrivis, C., Kitsiou, E., Stefanaki, S., Salamalekis, E., 2024, Isolated torsion of the fallopian tube: a case report and review of the literature. Clin Exp Obstet Gynecol. 31: 235–238.
[8]. Balasubramaniam, D., Duraisamy, K. Y., Ezhilmani, M., Ravi, S., 2020, Isolated Fallopian Tube Torsion: A Rare Twist with a Diagnostic Challenge That May Compromise Fertility. J Hum Reprod Sci;13: 162–167. doi:10.4103/jhrs.JHRS_143_19
[9]. Baumgartel, P. B., Fleischer, A. C., Cullinan, J. A., Bluth, R. F., 1996, Color Doppler sonography of tubal torsion. Ultrasound Obstet Gynecol. 7: 367–370. doi:10.1046/j.1469-0705.1996.07050367.x
[10]. Martín-Vallejo, J., Garrigós-Llabata, E. E., Molina-Bellido, P., Clemente-Pérez, P. A., 2020, Isolated fallopian tube torsion associated with hydrosalpinx in a 12-year-old girl: a case report. J Med Case Rep.14: 165. doi:10.1186/s13256-020-02462-1.
Viewed PDF 188 4 -
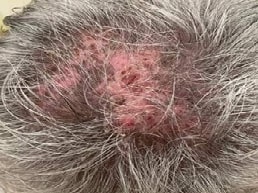 Dermoscopic and Histopathological Correlation in Cicatricial Alopecias: Unveiling Diagnostic and Therapeutic InsightsAuthor: Maghimaa MDOI: 10.21522/TIJPH.2013.SE.24.05.Art027
Dermoscopic and Histopathological Correlation in Cicatricial Alopecias: Unveiling Diagnostic and Therapeutic InsightsAuthor: Maghimaa MDOI: 10.21522/TIJPH.2013.SE.24.05.Art027Dermoscopic and Histopathological Correlation in Cicatricial Alopecias: Unveiling Diagnostic and Therapeutic Insights
Abstract:
Trichoscopy is dermoscopic imaging of the scalp and hair. It is a non-invasive proven technique that aids in the diagnosis of alopecia. It enhances the visualization of abnormalities of follicular ostia, perifollicular skin, hair shafts and cutaneous blood vessels. In cicatricial alopecia, loss of follicular openings, atrophy and scattered follicular pustules or single hair follicles can be identified. Prompt diagnosis and timely therapeutic intervention are of extreme importance as permanent hair loss can lead to disorders related to self-esteem and psychosocial interactions. Trichoscopy helps in early diagnosis, monitoring of disease progression and determining the response to therapy. To determine the efficacy of trichoscopy as a valuable and superior non-invasive method over histopathological examination to diagnose cicatricial alopecias. This is a cross-sectional study conducted in our hospital from September 2023 to November 2023. Patients with cicatricial alopecia were selected. Demographic data and clinical variables in terms of site of alopecia, type, duration, size and lesion morphology were documented. Heine delta 30 dermoscope was employed. Both polarized and non-polarized versions were used for examination. In this study, 16 patients were enrolled. The causes of cicatricial alopecia were traumatic alopecia (6), lichen planopilaris (5), discoid lupus erythematosus (4) and acne keloidalis nuchae (1). Dermoscopy facilitated the identification of unique and characteristic features that helped in prompt diagnosis. This was correlated with histopathological findings. Trichoscopy of cicatricial alopecia demonstrates characteristic patterns. It is an accurate diagnostic method that reduces the number of unnecessary biopsies and hence it is crucial for the diagnosis and follow-up of patients.
Dermoscopic and Histopathological Correlation in Cicatricial Alopecias: Unveiling Diagnostic and Therapeutic Insights
References:
[1]. Kanti, V., Röwert‐Huber, J., Vogt, A., Blume‐Peytavi, U., 2018. Cicatricial alopecia. JDDG: Journal der Deutschen Dermatologischen Gesellschaft, 16(4), 435-461.
[2]. Olsen, E. A., Bergfeld, W. F., Cotsarelis, G., Price, V. H., Shapiro, J., Sinclair, R., ... Whiting, D. A., 2003. Summary of North American Hair Research Society (NAHRS)-sponsored workshop on cicatricial alopecia, Duke university medical center, February 10 and 11, 2001. Journal of the American Academy of Dermatology, 48(1), 103-110.
[3]. Villablanca, S., Fischer, C., García-García, S. C., Mascaró-Galy, J. M., Ferrando, J., 2017. Primary scarring alopecia: clinical-pathological review of 72 cases and review of the literature. Skin Appendage Disorders, 3(3), 132-143.
[4]. Whiting, D. A., 2001. Cicatricial alopecia: clinico-pathological findings and treatment. Clinics in dermatology, 19(2), 211-225.
[5]. Tan, E., Martinka, M., Ball, N., Shapiro, J., 2004. Primary cicatricial alopecias: clinicopathology of 112 cases. Journal of the American Academy of Dermatology, 50(1), 25-32.
[6]. Jain, N., Doshi, B., Khopkar, U., 2013. Trichoscopy in alopecias: diagnosis simplified. International journal of trichology, 5(4), 170-178.
[7]. Tosti, A., 2007. Dermoscopy of Hair and Scalp Disorders: with Clinical and Pathological Correlations. CRC Press.
[8]. Puri, N., 2013. A clinical and histopathological study of cicatricial alopecia. Journal of Pakistan Association of Dermatologists, 23(3), 272-276.
[9]. Stefanato, C. M., 2010. Histopathology of alopecia: a clinicopathological approach to diagnosis. Histopathology, 56(1), 24-38.
[10]. Goyal, M., Khandpur, S., Ramam, M., Sharma, V. K., Singh, M. K., 2019. A study of the histopathological features of alopecias on transverse sections of scalp biopsies. Indian Journal of Dermatology, 64(1), 47-54.
[11]. Batchu, V. R., Panthalla, V. L., Kumar, P. K., Penchalaiah, K., 2022. A clinicopathological study on interface dermatitis. International Journal of Research, 8(5), 1.
[12]. Cummins, D. M., Chaudhry, I. H., Harries, M., 2021. Scarring alopecias: pathology and an update on digital developments. Biomedicines, 9(12), 1755.
[13]. Petak, A., Boras, J., Bata, I., Ilić, I., Hohšteter, M., Šoštarić‐Zuckermann, I. C., 2023. Clinical and histopathological investigation of symmetrical alopecia with associated chronic pruritus in tufted capuchin monkeys (Sapajus apella apella). Journal of Medical Primatology, 52(4), 244-258.
[14]. Thakur, B. K., Verma, S., Raphael, V., 2015. Clinical, trichoscopic, and histopathological features of primary cicatricial alopecias: A retrospective observational study at a tertiary care centre of North East India. International journal of trichology, 7(3), 107-112.
[15]. Trachsler, S., Trüeb, R. M., 2005. Value of direct immunofluorescence for differential diagnosis of cicatricial alopecia. Dermatology, 211(2), 98-102.
[16]. Olszewska, M., Rudnicka, L., Rakowska, A., Kowalska-Oledzka, E., Slowinska, M., 2008. Trichoscopy. Archives of Dermatology, 144(8), 1007-1007.
[17]. Jain, N., Doshi, B., Khopkar, U., 2013. Trichoscopy in alopecias: diagnosis simplified. International journal of trichology, 5(4), 170-178.
[18]. Sperling, L. C., Cowper, S. E., 2006. The histopathology of primary cicatricial alopecia. In Seminars in cutaneous medicine and surgery, 25(1), 41-50.
[19]. Pinedo-Moraleda, F., Tristán-Martín, B., & Dradi, G. G., 2023. Alopecias: practical tips for the management of biopsies and main diagnostic clues for general pathologists and dermatopathologists. Journal of Clinical Medicine, 12(15), 5004.
[20]. Umar, S., Ton, D., Carter, M. J., Shitabata, P., 2023. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clinical, Cosmetic and Investigational Dermatology, 2315-2327.
[21]. Palmer, V., Valdebran, M., 2023. Central centrifugal cicatricial alopecia in the adolescent population: an overview of available literature. Life, 13(4), 1022.
Viewed PDF 241 10 -
 Airway Management Using LMA‐Evaluation of Two Insertional Techniques: A Prospective Randomised StudyAuthor: Pradeep, KDOI: 10.21522/TIJPH.2013.SE.24.05.Art028
Airway Management Using LMA‐Evaluation of Two Insertional Techniques: A Prospective Randomised StudyAuthor: Pradeep, KDOI: 10.21522/TIJPH.2013.SE.24.05.Art028Airway Management Using LMA‐Evaluation of Two Insertional Techniques: A Prospective Randomised Study
Abstract:
The laryngeal mask airway (LMA), originally conceived as a component of anesthesiologists' airway armamentarium, has now become an indispensable airway adjunct for a broad spectrum of healthcare providers, extending to paramedics managing out-of-hospital cardiac arrest scenarios. his randomized trial compares the success rates of the traditional digital technique versus the 180-degree rotational technique for LMA insertion in patients undergoing superficial surgeries under general anesthesia. After ethical approval and informed consent, 120 healthy adults (ASA grade I-II, ages 18-65) scheduled for superficial surgeries were enrolled. Based on a pilot study, 52 cases per group were needed, with an additional 15% to account for dropouts, resulting in 60 participants per group. Exclusion criteria included emergency surgery, obesity, reflux disease, and procedures requiring prone positioning or lasting over an hour. No significant demographic or clinical differences were found between Group A (standard technique) and Group B (180-degree technique). The success rates were similar between the two techniques. However, the 180-degree technique may provide better oropharyngeal leak pressure, improving airway sealing and ventilation.
Airway Management Using LMA‐Evaluation of Two Insertional Techniques: A Prospective Randomised Study
References:
[1]. Pennant, J.H., Walker, M.B., 1992. Comparison of the Endotracheal Tube and Laryngeal Mask in Airway Management by Paramedical Personnel, Anesthesia & Analgesia,74, 531-534.
[2]. Eglen, M., Kuvaki, B., Günenç, F., Ozbilgin, S., Küçükgüçlü, S., Polat, E., et al., 2017. Comparação de três técnicas diferentes de inserção com a máscara laríngea LMA‐UniqueTM em adultos, resultados de um estudo randômico. Brazilian Journal of Anesthesiology, 67, 521–526.
[3]. Yu, S.H., Beirne, O.R.,2010. Laryngeal Mask Airways Have a Lower Risk of Airway Complications Compared With Endotracheal Intubation: A Systematic Review. Journal of Oral and Maxillofacial Surgery, 68, 2359–2376.
[4]. Haghighi, M., Mohammadzadeh, A., Naderi, B., Seddighinejad, A., Movahedi, H.,2010. Comparing two methods of LMA insertion; classic versus simplified (airway). Middle East J Anaesthesiol,20,509–514.
[5]. Brimacombe, J., Berry, A., 1993. Insertion of the Laryngeal Mask Airway—A Prospective Study of Four Techniques. Anaesth Intensive Care, 21, 89–92.
[6]. Hwang, J., Park, H.P., Lim, Y.J., Do, S.H., Lee, S.C., Jeon, Y.T., 2009. Comparison of Two Insertion Techniques of ProSealTM Laryngeal Mask Airway. Anesthesiology, 110, 905–907.
[7]. Asai, T., Brimacom, J., 2000. Cuff volume and size selection with the laryngeal mask. Anaesthesia, 55, 1179–1184.
[8]. Tan, Y., Jiang, J., Wan, R., 2022. Contrast of oropharyngeal leak pressure and clinical performance of I-gelTM and LMA ProSealTM in patients: A meta-analysis. PLoS ONE, 17, e0278871.
[9]. An, J., Shin, S.K., Kim, K.J., 2013. Laryngeal Mask Airway Insertion in Adults: Comparison between Fully Deflated and Partially Inflated Technique. Yonsei Med J, 54, 747.
[10]. Shyam, T., Selvaraj, V., 2021. Airway management using LMA-evaluation of three insertional techniques-a prospective randomised study. J Anaesthesiol Clin Pharmacol, 37,108.
[11]. Na, H.S., Jeon, Y.T, Shin, H.J., Oh, A.Y., Park, H.P., Hwang. J.W., 2015. Effect of Paralysis at the Time of ProSeal Laryngeal Mask Airway Insertion on Pharyngolaryngeal Morbidities. A Randomized Trial. Langevin SM. editor. PLoS ONE, 10, e0134130.
[12]. Koo, B.W., Oh, A.Y., Hwang, J.W., Na, H.S., Min, S.W., 2019. Comparison of standard versus 90° rotation technique for LMA FlexibleTM insertion: a randomized controlled trial. BMC Anesthesiol, 19, 95.
[13]. Soh, C.R., Ng, A.S.B., 2001. Laryngeal Mask Airway Insertion in Paediatric Anaesthesia: Comparison between the Reverse and Standard Techniques. Anaesth Intensive Care, 29, 515–519.
[14]. Nakayama, S., Osaka, Y., Yamashita. M., 2002. The rotational technique with a partially inflated laryngeal mask airway improves the ease of insertion in children. Pediatric Anesthesia, 12, 416–419.
[15]. Park, J.H., Lee, J.S., Nam, S.B., Ju, J.W., Kim, M.S., 2016. Standard versus Rotation Technique for Insertion of Supraglottic Airway Devices: Systematic Review and Meta-Analysis. Yonsei Med J,57,987.
[16]. Aghamohammadi, D., Eydi, M., Hosseinzadeh, H., Amiri Rahimi, M., Golzari, S., 2013. Assessment of Mini-dose Succinylcholine Effect on Facilitating Laryngeal Mask Airway Insertion. Journal of Cardiovascular and Thoracic Research, 5, 17-20.
[17]. Keller, C., Brimacombe, J., 1999. The Influence of Head and Neck Position on Oropharyngeal Leak Pressure and Cuff Position with the Flexible and the Standard Laryngeal Mask Airway. Anesthesia & Analgesia, 88, 913–916.
[18]. Dingley, J., Whitehead, S., Green, S., Bayley, E., 2009. A randomised controlled trial to compare the classic laryngeal mask airway with the i-gel airway in spontaneously breathing anaesthetised patients. Anaesthesia, 64, 674–678.
[19]. Yu, S.H., Beirne, O.R., 2010. Laryngeal Mask Airways Have a Lower Risk of Airway Complications Compared With Endotracheal Intubation: A Systematic Review. Journal of Oral and Maxillofacial Surgery, 68, 2359–2376.
[20]. Brain, A.I.J., McGhee, T.D., McAteer, E.J., Thomas, A., Abu-Saad, M.A., Bushman, J.A., 1985. The Laryngeal Mask Airway. Development and Preliminary Trials of a New Type of Airway. Anaesthesia, 40, 356–361.
Viewed PDF 179 3 -
 Computational Design of Bioactive Epigallocatechin Gallate (EGCG) Analogues Targeting Heme Oxygenase-1 (HO-1) Pathway for Metabolic RegulationAuthor: Sarvesh SabarathinamDOI: 10.21522/TIJPH.2013.SE.24.05.Art029
Computational Design of Bioactive Epigallocatechin Gallate (EGCG) Analogues Targeting Heme Oxygenase-1 (HO-1) Pathway for Metabolic RegulationAuthor: Sarvesh SabarathinamDOI: 10.21522/TIJPH.2013.SE.24.05.Art029Computational Design of Bioactive Epigallocatechin Gallate (EGCG) Analogues Targeting Heme Oxygenase-1 (HO-1) Pathway for Metabolic Regulation
Abstract:
Epigallocatechin-gallate (EGCG) is a flavone-based natural product that has a more significant impact on Diabetes and other cardiometabolic complications. This in silico based computer-aided drug design ensures the drug's Pharmacokinetic parameters and particular compounds towards the precise target. Based on the network data designed, EGCG were docked against PDB: 1N3U, using Dimethyl fumarate as the standard reference. Such desirable quality of modified EGCG will create a spark in the novel drug discovery of a bioenhancer. This slight evidence will support a higher quantum of drug discovery in semisynthetic chemistry toward Metabolic Complications.
Computational Design of Bioactive Epigallocatechin Gallate (EGCG) Analogues Targeting Heme Oxygenase-1 (HO-1) Pathway for Metabolic Regulation
References:
[2]. Tiwari S, Ndisang JF, 2014, The heme oxygenase system and type-1 diabetes, Current pharmaceutical design, 20(9):1328-37. Epub 2013/08/28. doi: 10.2174/13816128113199990552. PubMed PMID: 23978102.
[3]. Roberts MS, Burbelo PD, Egli-Spichtig D, Perwad F, Romero CJ, Ichikawa S, et al., 2018, Autoimmune hyperphosphatemic tumoral calcinosis in a patient with FGF23 autoantibodies, The Journal of clinical investigation, 128(12):5368-73. Epub 2018/09/19. doi: 10.1172/jci122004. PubMed PMID: 30226830; PubMed Central PMCID: PMCPMC6264742.
[4]. Pozzilli P, Pieralice S., 2018, Latent Autoimmune Diabetes in Adults: Current Status and New Horizons, Endocrinology and metabolism, 33(2):147-59. Epub 2018/06/28. doi: 10.3803/EnM.2018.33.2.147. PubMed PMID: 29947172; PubMed Central PMCID: PMCPMC6021307.
[5]. Paschou SA, Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C., 2018, On type 1 diabetes mellitus pathogenesis, Endocrine connections,7(1):R38-r46. Epub 2017/12/02. doi: 10.1530/ec-17-0347. PubMed PMID: 29191919; PubMed Central PMCID: PMCPMC5776665.
[6]. Rosenblum MD, Remedios KA, Abbas A K., 2015, Mechanisms of human autoimmunity, The Journal of clinical investigation, 125(6):2228-33. Epub 2015/04/22. doi: 10.1172/jci78088. PubMed PMID: 25893595; PubMed Central PMCID: PMCPMC4518692.
[7]. Atkinson MA, Maclaren NK., 1994, The pathogenesis of insulin-dependent diabetes mellitus, The New England journal of medicine, 331(21):1428-36. Epub 1994/11/24. doi: 10.1056/nejm199411243312107. PubMed PMID: 7969282.
[8]. Eizirik DL, Colli ML, Ortis F., 2009, The role of inflammation in insulitis and beta-cell loss in type 1 diabetes, Nature reviews Endocrinology, 5(4):219-26. Epub 2009/04/09. doi: 10.1038/nrendo.2009.21. PubMed PMID: 19352320.
[9]. Martin SJ, Green DR, 1995, Protease activation during apoptosis: Death by a thousand cuts?, Cell, 82(3):349-52. doi: https://doi.org/10.1016/0092-8674(95)90422-0.
[10]. Tomita T, 2017, Apoptosis of pancreatic β-cells in Type 1 diabetes, Bosnian journal of basic medical sciences, 17(3):183-93. Epub 2017/04/04. doi: 10.17305/bjbms.2017.1961. PubMed PMID: 28368239; PubMed Central PMCID: PMCPMC5581966.
[11]. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al,. 2020, Pathophysiology of Type 2 Diabetes Mellitus, International journal of molecular sciences, 21(17). Epub 2020/09/03. doi: 10.3390/ijms21176275. PubMed PMID: 32872570; PubMed Central PMCID: PMCPMC7503727.
[12]. Riddle MC, Cefalu WT, Evans PH, Gerstein HC, Nauck MA, Oh WK, et al., 2021, Consensus Report: Definition and Interpretation of Remission in Type 2 Diabetes, The Journal of Clinical Endocrinology & Metabolism, 107(1):1-9. doi: 10.1210/clinem/dgab585 %J The Journal of Clinical Endocrinology & Metabolism.
[13]. Shoelson SE, Lee J, Goldfine AB., 2006, Inflammation and insulin resistance, The Journal of clinical investigation, 116(7):1793-801. Epub 2006/07/11. doi: 10.1172/jci29069. PubMed PMID: 16823477; PubMed Central PMCID: PMCPMC1483173.
[14]. Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, et al., 2009, .Periadventitial adipose tissue plays a critical role in vascular remodeling, Circulation research, 105(9):906-11. Epub 2009/09/19. doi: 10.1161/circresaha.109.199653. PubMed PMID: 19762682.
[15]. Maliszewska K, Kretowski A., 2021, Brown Adipose Tissue and Its Role in Insulin and Glucose Homeostasis, Int J Mol Sci, 22(4):1530. PubMed PMID: doi:10.3390/ijms22041530.
[16]. Mileti E, Kwok KHM, Andersson DP, Mathelier A, Raman A, Bäckdahl J, et al., 2021, Human White Adipose Tissue Displays Selective Insulin Resistance in the Obese State, Diabetes , 70(7):1486-97. doi: 10.2337/db21-0001 %J Diabetes.
[17]. Tomita T, 2016, Apoptosis in pancreatic β-islet cells in Type 2 diabetes, Bosnian journal of basic medical sciences, 16(3):162-79. Epub 2016/05/23. doi: 10.17305/bjbms.2016.919. PubMed PMID: 27209071; PubMed Central PMCID: PMCPMC4978108.
[18]. Mitsuishi Y, Motohashi H, Yamamoto M, 2012, The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism, Frontiers in oncology, 2:200. Epub 2012/12/29. doi: 10.3389/fonc.2012.00200. PubMed PMID: 23272301; PubMed Central PMCID: PMCPMC3530133.
[19]. Kobayashi M, Yamamoto M, 2006, Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species, Advances in enzyme regulation, 46:113-40. Epub 2006/08/05. doi: 10.1016/j.advenzreg.2006.01.007. PubMed PMID: 16887173.
[20]. Kansanen E, Kuosmanen SM, Leinonen H, Levonen A L, 2013, The Keap1-Nrf2 pathwy: Mechanisms of activation and dysregulation in cancer, Redox biology, 1(1):45-9. Epub 2013/09/12. doi: 10.1016/j.redox.2012.10.001. PubMed PMID: 24024136; PubMed Central PMCID: PMCPMC3757665.
[21]. Canning P, Sorrell FJ, Bullock AN, 2015, Structural basis of Keap1 interactions with Nrf2, Free radical biology & medicine, 88(Pt B):101-7. Epub 2015/06/10. doi: 10.1016/j.freeradbiomed.2015.05.034. PubMed PMID: 26057936; PubMed Central PMCID: PMCPMC4668279.
[22]. Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, et al., 2015, Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease, Free radical biology & medicine, 88(Pt B):108-46. Epub 2015/07/01. doi: 10.1016/j.freeradbiomed.06.021. PubMed PMID: 26122708; PubMed Central PMCID: PMCPMC4659505.
[23]. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J., 2016, Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism, Cellular and Molecular Life Sciences, 73(17):3221-47. doi: 10.1007/s00018-016-2223-0.
[24]. Araujo J, Zhang M, Yin F, 2012, Heme Oxygenase-1, Oxidation, Inflammation, and Atherosclerosis, 3(01):01-14. doi: 10.3389/fphar.2012.00119.
[25]. Barañano DE, Ferris CD, Snyder SH, 2021, Atypical neural messengers, Trends in Neurosciences, 24(2):99-106. doi: https://doi.org/10.1016/S0166-2236(00)01716-1.
[26]. Rochette L, Zeller M, Cottin Y, Vergely C, 2018, Redox Functions of Heme Oxygenase-1 and Biliverdin Reductase in Diabetes, Trends in Endocrinology & Metabolism,29(2):74-85. doi: https://doi.org/10.1016/j.tem.2017.11.005.
[27]. Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH, 2009, Bilirubin and glutathione have complementary antioxidant and cytoprotective roles, Proc Natl Acad Sci U S A, 106(13):5171-6. doi: doi:10.1073/pnas.0813132106.
[28]. Seyoum A, Asres K, El-Fiky FK, 2006, Structure-radical scavenging activity relationships of flavonoids. Phytochemistry, 67(18):2058-70. Epub 2006/08/22. doi: 10.1016/j.phytochem.2006.07.002. PubMed PMID: 16919302.
[29]. Ru J, Li P, Wang J, Zhou W, Li B, Huang C, et al., 2014, TCMSP: a database of systems pharmacology for drug discovery from herbal medicines, Journal of Cheminformatics, 6(1):13. doi: 10.1186/1758-2946-6-13.
[30]. Ouassou H, Zahidi T, Bouknana S, Bouhrim M, Mekhfi H, Ziyyat A, et al., 2018, Inhibition of α-Glucosidase, Intestinal Glucose Absorption, and Antidiabetic Properties by Caralluma europaea, Evidence-based complementary and alternative medicine: eCAM, 9589472. Epub 2018/09/20. doi: 10.1155/2018/9589472. PubMed PMID: 30228829; PubMed Central PMCID: PMCPMC6136516.
[31]. Turner N, Zeng X-Y, Osborne B, Rogers S, Ye J-M, 2016, Repurposing Drugs to Target the Diabetes Epidemi, Trends in Pharmacological Sciences. 37(5):379-89. doi: https://doi.org/10.1016/j.tips.2016.01.007.
[32]. Wang B, Sun J, Li X, Zhou Q, Bai J, Shi Y, et al., 2013, Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet–induced obesity, Nutrition Research.33(11):971-81. doi: https://doi.org/10.1016/j.nutres.2013.07.016.
[33]. Song M-Y, Kim E-K, Moon W-S, Park J-W, Kim H-J, So H-S, et al., 2009, Sulforaphane protects against cytokine- and streptozotocin-induced β-cell damage by suppressing the NF-κB pathway. Toxicology and Applied Pharmacology. 235(1):57-67. doi: https://doi.org/10.1016/j.taap.2008.11.007.
[34]. Rashid K, Sil PC, 2015, Curcumin enhances recovery of pancreatic islets from cellular stress induced inflammation and apoptosis in diabetic rats, Toxicology and Applied Pharmacology, 282(3):297-310. doi: https://doi.org/10.1016/j.taap.2014.12.003.
[35]. Coskun O, Kanter M, Korkmaz A, Oter S, 2005, Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacological Research, 51(2):117-23. doi: https://doi.org/10.1016/j.phrs.2004.06.002.
[36]. Chen C-Y, Jang J-H, Li M-H, Surh Y-J, 2005, Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells, Biochemical and Biophysical Research Communications, 2005;331 (4):993-1000. doi: https://doi.org/10.1016/j.bbrc.2005.03.237.
[37]. Lin C-F, Chueh T-H, Chung C-H, Chung S-D, Chang T-C, Chien C-T, 2020., Sulforaphane improves voiding function via the preserving mitochondrial function in diabetic rats, Journal of the Formosan Medical Association, 119(9):1422-30. doi: https://doi.org/10.1016/j.jfma.2019.11.017.
[38]. Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, et al., 2009, Heme Oxygenase-1 Induction Remodels Adipose Tissue and Improves Insulin Sensitivity in Obesity-Induced Diabetic Rats, Hypertenssion, 53(3):508-15. doi: doi:10.1161/HYPERTENSIONAHA.108.124701.
[39]. Lim D-W, Kim H, Kim Y-M, Chin Y-W, Park W-H, Kim J-E, 2019, Drug repurposing in alternative medicine: herbal digestive Sochehwan exerts multifaceted effects against metabolic syndrome, Scientific Reports, 9(1):9055. doi: 10.1038/s41598-019-45099-x.
[40]. Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al., 2019, Drug repurposing: progress, challenges and recommendations, Nature Reviews Drug Discovery, 18(1):41-58. doi: 10.1038/nrd.2018.168.
[41]. Gonzalez-Alfonso JL, Peñalver P, Ballesteros AO, Morales JC, Plou FJ, 2019, Effect of α-Glucosylation on the Stability, Antioxidant Properties, Toxicity, and Neuroprotective Activity of (–)-Epigallocatechin Gallate, 6(1):1-10. doi: 10.3389/fnut.2019.00030.
[42]. Hong Z, Xu Y, Yin J-F, Jin J, Jiang Y, Du Q, 2014, Improving the Effectiveness of (−)-Epigallocatechin Gallate (EGCG) against Rabbit Atherosclerosis by EGCG-Loaded Nanoparticles Prepared from Chitosan and Polyaspartic Acid, Journal of Agricultural and Food Chemistry, 62(52):12603-9. doi: 10.1021/jf504603n.
[43]. Sharifi-Rad M, Pezzani R, Redaelli M, Zorzan M, Imran M, Ahmed Khalil A, et al., 2020, Preclinical Activities of Epigallocatechin Gallate in Signaling Pathways in Cancer, Molecules, 25(3):467. PubMed PMID: doi:10.3390/molecules25030467.
[44]. Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH, Wung BS, 2006, Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways, Life Sciences, 78(25):2889-97. doi: https://doi.org/10.1016/j.lfs.2005.11.013.
[45]. Zhou T, Zhu M, Liang Z, 2018, (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson's disease, Mol Med Rep, 17(4):4883-8. doi: 10.3892/mmr.2018.8470.
[46]. Irfan, M, Lee, YY, Lee, KJ, Kim, SD, & Rhee, MH, 2022, Comparative antiplatelet and antithrombotic effects of red ginseng and fermented red ginseng extracts, Journal of Ginseng Research, 46(3), 387-395.
[47]. Singla, N, Gupta, G, Kulshrestha, R, Sharma, K, Bhat, AA, Mishra, R, & Gupta, S, 2024, Daidzein in Traditional Chinese Medicine: A Deep Dive into Its Ethnomedicinal and Therapeutic Applications, Pharmacological Research-Modern Chinese Medicine, 100460.
[48]. Wang, J., Behl, T., Rana, T., Sehgal, A., Wal, P., Saxena, B., & Singla, R. K., 2024, Exploring the Pathophysiological Influence of Heme Oxygenase-1 on Neuroinflammation and Depression: A Study of Phytotherapeutic-Based Modulation, Phytomedicine, 155466.
Viewed PDF 153 0 -
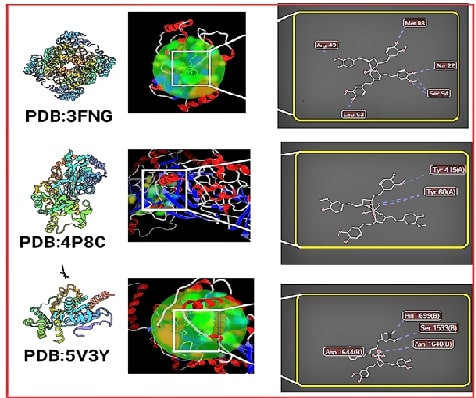 Curcumin Versus Curcumin-Iron Complex Docking in Tuberculosis Targets: A Insights on Synthesis & Molecular Docking StudyAuthor: Sarvesh SabarathinamDOI: 10.21522/TIJPH.2013.SE.24.05.Art030
Curcumin Versus Curcumin-Iron Complex Docking in Tuberculosis Targets: A Insights on Synthesis & Molecular Docking StudyAuthor: Sarvesh SabarathinamDOI: 10.21522/TIJPH.2013.SE.24.05.Art030Curcumin Versus Curcumin-Iron Complex Docking in Tuberculosis Targets: A Insights on Synthesis & Molecular Docking Study
Abstract:
In this contemporary state Natural products play a significant role in drug discovery. This research investigates a Curcumin-Iron complex (CuFe) as a potential new weapon against tuberculosis (TB). The study highlights the global burden of TB and the need for alternatives due to rising drug resistance. Curcumin, a promising compound from turmeric, suffers from poor absorption in the body. The researchers created CuFe and confirmed its structure using XRD analysis to address this. Using computer simulations, they then tested CuFe's binding to key targets in the TB bacteria. Excitingly, CuFe showed superior binding compared to curcumin and existing drugs. The discussion emphasizes the potential of plant-based medicines like curcumin, along with metal complexes, mentioning garlic and piperine as further avenues. The authors conclude that CuFe is a promising candidate, but further testing in animals and humans, along with studies on absorption and regulatory approval, is needed before it can be considered a viable TB treatment.
Curcumin Versus Curcumin-Iron Complex Docking in Tuberculosis Targets: A Insights on Synthesis & Molecular Docking Study
References:
[1]. Oryema, C., Rutaro, K., Oyet, S.W., and Malinga, G.M., 2021. Ethnobotanical plants used in the management of symptoms of tuberculosis in rural Uganda. Tropical Medicine and Health.; 49, 92. 10.1186/s41182-021-00384-2.
[2]. Mangwani, N., Singh, P.K., and Kumar, V., 2020. Medicinal plants: Adjunct treatment to tuberculosis chemotherapy to prevent hepatic damage. Journal of Ayurveda and integrative medicine.; 11, 522-528. 10.1016/j.jaim.2019.02.004.
[3]. Terreni, M., Taccani, M., and Pregnolato, M.,2021. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules (Basel, Switzerland).; 26. 10.3390/molecules26092671.
[4]. Sharifi-Rad, J., Rayess, Y.E., Rizk, A.A., Sadaka, C., Zgheib, R., Zam, W., Sestito, S., Rapposelli, S., Neffe-Skocińska, K., Zielińska, D., et al., 2020. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Frontiers in pharmacology.; 11, 01021. 10.3389/fphar.2020.01021.
[5]. Iweala, E.J., Uche, M.E., Dike, E.D., Etumnu, L.R., Dokunmu, T.M., Oluwapelumi, A.E., Okoro, B.C., Dania, O.E., Adebayo, A.H., and Ugbogu, E.A., 2023. Curcuma longa (Turmeric): Ethnomedicinal uses, phytochemistry, pharmacological activities and toxicity profiles—A review. Pharmacological Research - Modern Chinese Medicine .; 6, 100222. https://doi.org/10.1016/j.prmcm.2023.100222.
[6]. Naksuriya, O., Okonogi, S., Schiffelers, R.M., and Hennink, W.E., 2014. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials.; 35, 3365-3383. https://doi.org/10.1016/j.biomaterials.2013.12.090.
[7]. Idoudi, S., Bedhiafi, T., Hijji, Y.M., and Billa, N., 2022. Curcumin and Derivatives in Nanoformulations with Therapeutic Potential on Colorectal Cancer. AAPS PharmSciTech.; 23, 115. 10.1208/s12249-022-02268-y.
[8]. Iranshahi, M., Chini, M.G., Masullo, M., Sahebkar, A., Javidnia, A., Chitsazian Yazdi, M., Pergola, C., Koeberle, A., Werz, O., Pizza, C., et al., 2015. Can Small Chemical Modifications of Natural Pan-inhibitors Modulate the Biological Selectivity? The Case of Curcumin Prenylated Derivatives Acting as HDAC or mPGES-1 Inhibitors. Journal of Natural Products 78, 2867-2879. 10.1021/acs.jnatprod.5b00700.
[9]. Delpu, Y., Cordelier, P., Cho, W.C., and Torrisani, J., 2013. DNA Methylation and Cancer Diagnosis. 14, 15029-15058.
[10]. Afzal, O., Yusuf, M., Ahsan, M.J., Altamimi, A.S.A., Bakht, M.A., Ali, A., and Salahuddin., 2022. Chemical Modification of Curcumin into Its Semi-Synthetic Analogs Bearing Pyrimidinone Moiety as Anticancer Agents. 11, 2737.
[11]. Venkatesan, P., and Rao, M.N.A., 2010. Structure–Activity Relationships for the Inhibition of Lipid Peroxidation and the Scavenging of Free Radicals by Synthetic Symmetrical Curcumin Analogues. Journal of Pharmacy and Pharmacology 52, 1123-1128. 10.1211/0022357001774886 %J Journal of Pharmacy and Pharmacology.
[12]. Hatamie, S., Nouri, M., Karandikar, S.K., Kulkarni, A., Dhole, S.D., Phase, D.M., and Kale, S.N., 2012. Complexes of cobalt nanoparticles and polyfunctional curcumin as antimicrobial agents. Materials Science and Engineering: C 32, 92-97. https://doi.org/10.1016/j.msec.2011.10.002.
[13]. Refat, M.S., 2013. Synthesis and characterization of ligational behavior of curcumin drug towards some transition metal ions: Chelation effect on their thermal stability and biological activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 105, 326-337. https://doi.org/10.1016/j.saa.2012.12.041.
[14]. Sun, X., Tsang, C.-N., and Sun, H., 2008. Identification and characterization of metallodrug binding proteins by (metallo)proteomics†. Metallomics 1, 25-31. 10.1039/b813121j %J Metallomics.
[15]. Vaithiyalingam, M., Sumathi, D.L., and Sabarathinam, S., 2023. Isolation and In silico Study of Curcumin from Curcuma longa and Its Anti-Diabetic Activity. Applied biochemistry and biotechnology 195, 947-957. 10.1007/s12010-022-04173-3.
[16]. Bicer, N., Yildiz, E., Yegani, A.A., and Aksu, F., 2018. Synthesis of curcumin complexes with iron(iii) and manganese(ii), and effects of curcumin–iron(iii) on Alzheimer's disease. New Journal of Chemistry 42, 8098-8104. 10.1039/C7NJ04223J.
[17]. Vaithiyalingam, M., Mohan Kumar, R., Khagar, P., Sabarathinam, S., Alghazwani, Y., and Chidambaram, K., 2024. Isolation of 6-gingerol and semi-synthesis of 1,4-benzodiazepines derivatives: An in-situ pharmacokinetics properties, molecular docking and molecular dynamics simulation assessments. Saudi Journal of Biological Sciences 31, 104048. 10.1016/j.sjbs.2024.104048.
[18]. Sabarathinam, S., Ganamurali, N., Satheesh, S., Dhanasekaran, D., and Raja, A., 2023. Pharmacokinetic correlation of structurally modified chalcone derivatives as promising leads to treat tuberculosis. Future Medicinal Chemistry. 10.4155/fmc-2023-0161.
[19]. Yang, X., Liu, Y., Gan, J., Xiao, Z.-X., and Cao, Y., 2022. FitDock: protein–ligand docking by template fitting. Briefings in Bioinformatics 23. 10.1093/bib/bbac087.
[20]. Liu, Y., Grimm, M., Dai, W.T., Hou, M.C., Xiao, Z.X., and Cao, Y., 2020. CB-Dock: a web server for cavity detection-guided protein-ligand blind docking. Acta pharmacologica Sinica 41, 138-144. 10.1038/s41401-019-0228-6.
[21]. Liu, Y., Yang, X., Gan, J., Chen, S., Xiao, Z.-X., and Cao, Y., 2022. CB-Dock2: improved protein–ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Research 50, W159-W164. 10.1093/nar/gkac394 %J Nucleic Acids Research.
[22]. Hartkoorn, R.C., Sala, C., Neres, J., Pojer, F., Magnet, S., Mukherjee, R., Uplekar, S., Boy-Röttger, S., Altmann, K.-H., and Cole, S.T., 2012. Towards a new tuberculosis drug: pyridomycin – nature's isoniazid. 4, 1032-1042. https://doi.org/10.1002/emmm.201201689.
[23]. Rozwarski, D.A., Vilchèze, C., Sugantino, M., Bittman, R., and Sacchettini, J.C., 1999. Crystal Structure of the Mycobacterium tuberculosis Enoyl-ACP Reductase, InhA, in Complex with NAD+ and a C16 Fatty Acyl Substrate*. Journal of Biological Chemistry 274, 15582-15589. https://doi.org/10.1074/jbc.274.22.15582.
[24]. Freundlich, J.S., Wang, F., Vilchèze, C., Gulten, G., Langley, R., Schiehser, G.A., Jacobus, D.P., Jacobs Jr., W.R., and Sacchettini, J.C., 2009. Triclosan Derivatives: Towards Potent Inhibitors of Drug-Sensitive and Drug-Resistant Mycobacterium tuberculosis. 4, 241-248. https://doi.org/10.1002/cmdc.200800261.
[25]. Neres, J., Hartkoorn, R.C., Chiarelli, L.R., Gadupudi, R., Pasca, M.R., Mori, G., Venturelli, A., Savina, S., Makarov, V., Kolly, G.S., et al., 2015. 2-Carboxyquinoxalines Kill Mycobacterium tuberculosis through Noncovalent Inhibition of DprE1. ACS Chemical Biology 10, 705-714. 10.1021/cb5007163.
[26]. Aggarwal, A., Parai, M.K., Shetty, N., Wallis, D., Woolhiser, L., Hastings, C., Dutta, N.K., Galaviz, S., Dhakal, R.C., Shrestha, R., et al., 2017. Development of a Novel Lead that Targets M. tuberculosis Polyketide Synthase 13. Cell 170, 249-259.e225. https://doi.org/10.1016/j.cell.2017.06.025.
[27]. Nikiforov, P.O., Surade, S., Blaszczyk, M., Delorme, V., Brodin, P., Baulard, A.R., Blundell, T.L., and Abell, C., 2016. A fragment merging approach towards the development of small molecule inhibitors of Mycobacterium tuberculosis EthR for use as ethionamide boosters. Organic & Biomolecular Chemistry 14, 2318-2326. 10.1039/C5OB02630J.
[28]. Willand, N., Dirié, B., Carette, X., Bifani, P., Singhal, A., Desroses, M., Leroux, F., Willery, E., Mathys, V., Déprez-Poulain, R., et al., 2009. Synthetic EthR inhibitors boost antituberculous activity of ethionamide. Nature Medicine 15, 537-544. 10.1038/nm.1950.
[29]. Zhang, Z., Bulloch, E.M., Bunker, R.D., Baker, E.N., and Squire, C.J. 2009., Structure and function of GlmU from Mycobacterium tuberculosis. Acta crystallographica. Section D, Biological crystallography 65, 275-283. 10.1107/s0907444909001036.
[30]. Kumar, M., Singh, S.K., Singh, P.P., Singh, V.K., Rai, A.C., Srivastava, A.K., Shukla, L., Kesawat, M.S., Kumar Jaiswal, A., Chung, S.M., and Kumar, A., 2021. Potential Anti-Mycobacterium tuberculosis Activity of Plant Secondary Metabolites: Insight with Molecular Docking Interactions. Antioxidants (Basel, Switzerland) 10. 10.3390/antiox10121990.
[31]. Scarim, C.B., Lira de Farias, R., Vieira de Godoy Netto, A., Chin, C.M., Leandro dos Santos, J., and Pavan, F.R., 202. Recent advances in drug discovery against Mycobacterium tuberculosis: Metal-based complexes. European Journal of Medicinal Chemistry 214, 113166. https://doi.org/10.1016/j.ejmech.2021.113166.
[32]. Mjos, K.D., and Orvig, C., 2014. Metallodrugs in Medicinal Inorganic Chemistry. Chemical Reviews .; 114, 4540-4563. 10.1021/cr400460s.
[33]. Pawar, A., Jha, P., Chopra, M., Chaudhry, U., and Saluja, D., 2020. Screening of natural compounds that targets glutamate racemase of Mycobacterium tuberculosis reveals the anti-tubercular potential of flavonoids. Scientific Reports.; 10, 949. 10.1038/s41598-020-57658-8.
[34]. Sarangi, A., Das, B.S., Patnaik, G., Sarkar, S., Debnath, M., Mohan, M., and Bhattacharya, D.,2021. Potent anti-mycobacterial and immunomodulatory activity of some bioactive molecules of Indian ethnomedicinal plants that have the potential to enter in TB management. Journal of applied microbiology., 131, 1578-1599. 10.1111/jam.15088.
[35]. Irfan, M., Lee, Y. Y., Lee, K. J., Kim, S. D., & Rhee, M. H., 2022. Comparative antiplatelet and antithrombotic effects of red ginseng and fermented red ginseng extracts. Journal of Ginseng Research, 46(3), 387-395.
[36]. Singla, N., Gupta, G., Kulshrestha, R., Sharma, K., Bhat, A. A., Mishra, R., Gupta, S., 2024. Daidzein in Traditional Chinese Medicine: A Deep Dive into Its Ethnomedicinal and Therapeutic Applications. Pharmacological Research-Modern Chinese Medicine, 12 (01) 100460.
[37]. Wang, J., Behl, T., Rana, T., Sehgal, A., Wal, P., Saxena, B., ... & Singla, R. K., 2024. Exploring the Pathophysiological Influence of Heme Oxygenase-1 on Neuroinflammation and Depression: A Study of Phytotherapeutic-Based Modulation. Phytomedicine,; 127 (07)155466.
Viewed PDF 206 7 -
 Anaesthetic Management of A Patient with Pan Facial Trauma with Restricted Mouth Opening Posted for Open Reduction and Internal Fixation of Facial Bone FracturesAuthor: Mahalakshmi RDOI: 10.21522/TIJPH.2013.SE.24.05.Art031
Anaesthetic Management of A Patient with Pan Facial Trauma with Restricted Mouth Opening Posted for Open Reduction and Internal Fixation of Facial Bone FracturesAuthor: Mahalakshmi RDOI: 10.21522/TIJPH.2013.SE.24.05.Art031Anaesthetic Management of A Patient with Pan Facial Trauma with Restricted Mouth Opening Posted for Open Reduction and Internal Fixation of Facial Bone Fractures
Abstract:
Airway management of patients with maxillofacial trauma remains a challenging task for an anaesthesiologist in emergency and perioperative settings due to anatomical distortion. Appropriate planning and a team based approach is mandatory for establishing the airway during elective surgical procedures and in postoperative period. Here we report a 24 year old male with alleged history of road traffic accident who sustained injury to face. Patient had restricted mouth opening of 1 finger breadth due to pain and multiple facial bone fractures. Patient was posted for Open reduction and Internal fixation of facial bone fractures. In view of anticipated difficult intubation airway management was discussed as team and planned. In OR, before induction, USG guided bilateral maxillary and mandibular block was given, which improved patient’s mouth opening. This approach facilitated easy intubation and thereby avoiding airway related complication. The tube was fixed submentally so as to allow good intraoral work space for the surgeons. This case report suggests that use of USG guided maxillary and mandibular blocks facilitated in airway management in this patient with restricted mouth opening. Proper preemptive planning for difficult airway cases and multimodal analgesia helps in managing facial trauma cases successfully without complications.
Anaesthetic Management of A Patient with Pan Facial Trauma with Restricted Mouth Opening Posted for Open Reduction and Internal Fixation of Facial Bone Fractures
References:
[1]. Kovacs, G., & Sowers, N. 2018, Airway management in trauma. Emergency Medicine Clinics of North America, 36(1), 61–84.
[2]. Saini, S., Singhal, S., & Prakash, S. 2021, Airway management in maxillofacial trauma. Journal of Anaesthesiology, Clinical Pharmacology, 37(3), 319–327.
[3]. Nordquist, D., & Halaszynski, T. M. 2014, Perioperative multimodal anesthesia using regional techniques in the aging surgical patient. Pain Research and Treatment, 2014, 902174.
[4]. Barak, M., Bahouth, H., Leiser, Y., & Abu El-Naaj, I. 2015, Airway management of the patient with maxillofacial trauma: Review of the literature and suggested clinical approach. BioMed Research International, 2015, 724032.
[5]. Dhanrajani, P. J., & Jonaidel, O. 2002, Trismus: aetiology, differential diagnosis and treatment. Dental Update, 29(2), 88–92, 94.
[6]. Kassam, K., & Messiha, A. 2014, Fractured zygomatic arch: a traumatic cause for trismus. BMJ Case Reports, 2014(apr03 1), bcr2013202633.
[7]. Kojima, Y., Sendo, R., Ohno, S., & Sugimura, M. 2020, Ultrasound-guided inferior alveolar nerve block for trismus during dental treatment: a case report. JA Clinical Reports, 6(1), 94.
[8]. Heard, A. M. B., Green, R. J., Lacquiere, D. A., & Sillifant, P. 2009, The use of mandibular nerve block to predict safe anaesthetic induction in patients with acute trismus. Anaesthesia, 64(11), 1196–1198.
[9]. Jain, G., Yadav, G., Singh, A. P., Singh, Y., & Singh, D. K. 2016, Efficacy of ultrasound-guided mandibular block in predicting safer anesthetic induction. Anesthesia, Essays and Researches, 10(2), 184–188.
[10]. Shetty, P. M., Yadav, S. K., & Upadya, M. 2011, Submental intubation in patients with panfacial fractures: A prospective study. Indian Journal of Anaesthesia, 55(3), 299–304.
[11]. Borba, A. M., Porto, A. N., Santini, A., Santos, T. I. dos, Miloro, M., Borges, A. H., & Pedro, F. L. M. 2018, The effect of facial fractures on mouth opening range: a case series. RSBO, 1(3), 142.
[12]. Kumita, S., Murouchi, T., & Arakawa, J. 2017, Ultrasound-guided maxillary and inferior alveolar nerve blocks for postoperative analgesia in gnathoplasty. Asian Journal of Anesthesiology, 55(4), 89–90.
[13]. Sola, C., Raux, O., Savath, L., Macq, C., Capdevila, X., & Dadure, C. 2012, Ultrasound guidance characteristics and efficiency of suprazygomatic maxillary nerve blocks in infants: a descriptive prospective study: Ultrasound-guided suprazygomatic maxillary nerve block. Paediatric Anaesthesia, 22(9), 841–846.
Viewed PDF 197 6 -
 Distinguishing between Emergence Delirium and Pain in Early Post-Operative Period among Paediatric Patients: A Prospective Observational StudyAuthor: Anand SDOI: 10.21522/TIJPH.2013.SE.24.05.Art032
Distinguishing between Emergence Delirium and Pain in Early Post-Operative Period among Paediatric Patients: A Prospective Observational StudyAuthor: Anand SDOI: 10.21522/TIJPH.2013.SE.24.05.Art032Distinguishing between Emergence Delirium and Pain in Early Post-Operative Period among Paediatric Patients: A Prospective Observational Study
Abstract:
Emergence delirium (ED) and postoperative pain are common and significant concerns in paediatric anaesthesia. Both conditions can occur early in the postoperative period and are challenging to distinguish due to overlapping clinical presentations. ED is characterized by confusion, agitation, and disorientation after anaesthesia, often leading to distress in the child and anxiety among caregivers. Postoperative pain can similarly present with agitation and crying, making differentiation critical for effective management. This study aimed to prospectively observe paediatric patients to identify distinguishing characteristics between ED and pain, thereby improving postoperative care and reducing misdiagnosis. This prospective observational study included 100 paediatric patients aged 2 to 6 years. All participants were ASA physical status 1 or 2 and underwent elective surgeries requiring general anaesthesia. Postoperative behaviour was assessed using the PAED score for ED and the FLACC scale for pain. Two trained observers, blinded to the study hypothesis, evaluated the children at multiple time points during and after surgery. The primary outcomes were the incidence of ED and pain, and the relationship between sevoflurane exposure time and these outcomes. The majority of patients (89.375%) exhibited normal postoperative behaviour, while 10.5% experienced ED, and 3.75% experienced pain without ED. A smaller subset (3.5%) experienced both ED and pain. Patients with sevoflurane exposure greater than 100 minutes had a significantly higher risk of ED, with a risk ratio of 4.5 compared to those with less exposure. ED was most prevalent at awakening and rapidly decreased, while pain became more prominent later in the recovery period. While most paediatric patients recover from anaesthesia without issues, a notable group remains at risk for ED, especially with longer sevoflurane exposure.
Distinguishing between Emergence Delirium and Pain in Early Post-Operative Period among Paediatric Patients: A Prospective Observational Study
References:
[1]. Bai, Y., Jin, Q., Cai, W., Li, J., Zhou, Y., 2024., Effects of the timing of satisfactory sedation with preoperative oral midazolam on anesthesia induction and recovery in children undergoing adenotonsillectomy., Chinese Journal of Clinical Medicine., 29(3):296.
[2]. Maaly AM, Mahgoub A, Osman Y, et al. 2024, Comparison between nalbuphine versus dexmedetomidine for prevention of emergence agitation in pediatrics during sevoflurane anesthesia: prospective study. Alexandria Journal of Medicine. 60(3):237-244.
[3]. Hassan MAI, Tawfik MS, El Desouky MI, et al. 2024, Effects of Preoperative Ketamine or Midazolam or Ketamine versus Oral Dextromethorphan for Reducing Sevoflurane Emergence Agitation among Preschool Children. Zagazig University Medical Journal.
[4]. Davies L, Qi TS, Ng A. 2024, Emergence delirium: an overview with an emphasis on the use of electroencephalography in its management. Anesthesia and Pain Medicine.
[5]. Sawicki, CM., Janal, MN., Wade, SD., 2024., Preoperative Multisensory Room Use in Pediatric Patients with Autism: A Randomized Clinical Trial. Pediatric Dentistry.,46(2):123-130.
[6]. Hajdini H, Guelbert C, Sanofky B, Spencer J. 2024, Implementation of Pediatric Emergence Delirium (PAED) Scale. Journal of PeriAnesthesia Nursing.
[7]. Zenghui, Liang., Yanle, Xie., Shuhan, Chen., Haibo, Liu., Lv, H., Bertrand-Geoffrey, Muhoza., Fei, Xing., Jingjing, Yuan., Wei, Xin., Na, Xing., Jianjun, Yang., Z, Wang., Jingjing, Yuan. 2024, Predicting postoperative pain in children: an observational study using the pain threshold Index. Frontiers in Pediatrics, doi: 10.3389/fped.2024.1398182.
[8]. Jian-Ming, Yue., Qi, Wang., Bin, Liu., Leng, Zhou. 2024, Postoperative accurate pain assessment of children and artificial intelligence: A medical hypothesis and planned study. World Journal of Clinical Cases, (2024). doi: 10.12998/wjcc.v12.i4.681.
[9]. Sugandhini, Boda., Rajeshwari, K. 2024, Association between anesthetic type and perioperative outcomes in pediatric patients undergoing elective surgery: An observational study. Asian journal of medical sciences, doi: 10.3126/ajms.v15i2.59580.
[10]. Lijing, Li., Jianmin, Zhang., Jiayi, Li., Yi, Ren., Zheng, Gao., Jia, Gao., Fuzhou, Zhang., Fang, Wang., Tiehua, Zheng. 2023, Development of a nomogram to predict negative postoperative behavioral changes based on a prospective cohort. BMC Anesthesiology, doi: 10.1186/s12871-023-02228-4.
[11]. Semkovych, Ya.V. 2023, Clinical and laboratory changes in postsurgical pain markers in children. Neonatology surgery and perinatal medicine 8(4(50)):92-98, (2023), doi: 10.24061/2413-4260.xiii.4.50.2023.13.
[12]. Aouad, M. T., & Nasr, V. G. 2007, Emergence agitation in children: An update. Current Opinion in Anaesthesiology, 20(4), 367-373.
[13]. Sikich, N., & Lerman, J. 2004, Development and psychometric evaluation of the Pediatric Anesthesia Emergence Delirium Scale. Anesthesiology, 100(5), 1138-1145.
[14]. Chandler, J. R., Myers, D., Mehta, D., et al. 2008, Emergence delirium in children: A randomized trial to compare total intravenous anesthesia with sevoflurane anesthesia. Pediatric Anesthesia, 18(4), 332-340.
[15]. Dahmani, S., Delivet, H., & Hilly, J. 2010, Emergence delirium in children: An update. Current Opinion in Anaesthesiology, 23(4), 494-499.
[16]. Moore, A. D., Anghelescu, D. L., Christensen, R., et al. 2010. Emergence delirium in pediatric patients: Risk factors and associated behaviors. Journal of Pediatric Surgery, 45(4), 714-718.
[17]. Sarah, R, Martin., Theodore, W., Heyming., Michelle, A., Fortier., Zeev, N., Kain. (1) Paediatric laceration repair in the emergency department: post-discharge pain and maladaptive behavioural changes. Emergency Medicine Journal, (2024). doi: 10.1136/emermed-2023-213858.
[18]. Rabbitts, J. A., Fisher, E., Rosenbloom, B. N., & Palermo, T. M. (2017). Prevalence and predictors of chronic postsurgical pain in children: a systematic review and meta-analysis. The journal of pain, 18(6), 605-614, doi: https://doi.org/10.1016/j.jpain.2017.03.007.
[19]. Donna, Eull., Wendy, S., Looman., Susan, K, O'Conner-Von. 2023, Transforming acute pain management in children: A concept analysis to develop a new model of nurse, child and parent partnership. Journal of Clinical Nursing, doi: 10.1111/jocn.16625.
[20]. Postoperative Pain in Pediatrics. doi: 10.5772/intechopen.111788.
Viewed PDF 205 2 -
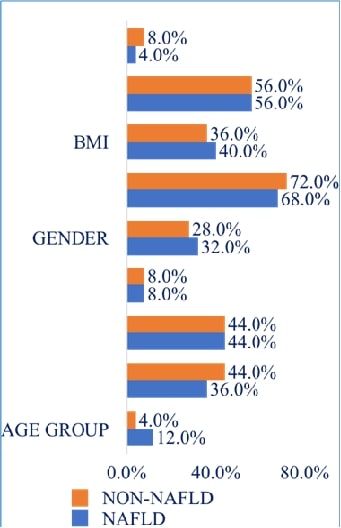 Association of Microalbuminuria in Patient with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in A Tertiary Care CentreAuthor: Maghimaa MDOI: 10.21522/TIJPH.2013.SE.24.05.Art033
Association of Microalbuminuria in Patient with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in A Tertiary Care CentreAuthor: Maghimaa MDOI: 10.21522/TIJPH.2013.SE.24.05.Art033Association of Microalbuminuria in Patient with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in A Tertiary Care Centre
Abstract:
NAFLD (Nonalcoholic Fatty Liver Disease) is a prevalent chronic liver disorder characterized by metabolic abnormalities. Microalbuminuria have been linked to cardiovascular and renal diseases, but its association with NAFLD remains underexplored. This cross-sectional study assessed urine microalbumin levels in patients diagnosed with NAFLD. Demographic data, NAFLD grading, and microalbuminuria status were analyzed in non-NAFLD and NAFLD groups. Statistical analysis has been performed to evaluate the associations. Males were more common than females among NAFLD patients (n=100), particularly in the age range of 41 to 60. Compared to non-NAFLD controls (0%), NAFLD patients (76%) had a considerably higher prevalence of microalbuminuria. BMI showed no significant association with NAFLD prevalence. NAFLD grading revealed Grade 1 as the most prevalent (52%), followed by Grade 2 (32%) and Grade 3 (16%). Our findings demonstrate a strong association between microalbuminuria and NAFLD, suggesting a potential link with adverse cardiovascular and renal outcomes. Monitoring microalbuminuria in NAFLD patients may aid in risk stratification and management. There is a need to conduct larger population-based-randomized studies.
Association of Microalbuminuria in Patient with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in A Tertiary Care Centre
References:
[1]. Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59: 859–871.
[2]. Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J Gastroenterol. 2018;24: 3361–3373.
[3]. Lr SG, Dhande SK, Kv R, M J. Correlation between severity of thrombocytopenia and portal hypertensive gastropathy in patients with chronic liver disease. J Assoc Physicians India. 2023;71: 1.
[4]. Kadiyala V, Savitha G. Assessment of Gamma Glutamyl Transferase in Chronic Periodontitis. Research Journal of Pharmacy and Technology. 2018;11: 5083–5085.
[5]. Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig Liver Dis. 2017;49: 471–483.
[6]. Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J Hepatol. 2018;68: 335–352.
[7]. Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A. 2006;103: 18273–18277.
[8]. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64: 73–84.
[9]. Tha TP, Saveetha Dental College and Hospital, Chennai, Tamilnadu, India. Non-pharmacological treatment for non alcoholic fatty liver DiseaseA review. J Med Sci Clin Res. 2016. doi:10.18535/jmscr/v4i8.61.
[10]. Lee YH, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, et al. Nonalcoholic fatty liver disease in diabetes. Part I: Epidemiology and diagnosis. Diabetes Metab J. 2019;43: 31–45.
[11]. Han E, Kim MK, Jang BK, Kim HS. Albuminuria is associated with steatosis burden in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Diabetes Metab J. 2021;45: 698–707.
[12]. Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47: 181–190.
[13]. Ahn SH, Seo DH, Kim SH, Nam M-S, Hong S. The relationship between fatty liver index and bone mineral density in Koreans: KNHANES 2010–2011. Osteoporos Int. 2018;29: 181–190.
[14]. Petta S, Valenti L, Bugianesi E, Targher G, Bellentani S, Bonino F, et al. A “systems medicine” approach to the study of non-alcoholic fatty liver disease. Dig Liver Dis. 2016;48: 333–342.
[15]. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65: 589–600.
[16]. Becker GJ, Wheeler DC, De ZD, Fujita T, Furth SL, Holdaas H, et al. Kidney disease: Improving global outcomes (KDIGO) blood pressure work group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney International Supplements. 2012;2: 337–414.
[17]. Lieb W, Mayer B, Stritzke J, Doering A, Hense H-W, Loewel H, et al. Association of low-grade urinary albumin excretion with left ventricular hypertrophy in the general population: the MONICA/KORA Augsburg Echocardiographic Substudy. Nephrol Dial Transplant. 2006;21: 2780–2787.
[18]. Tanaka F, Komi R, Makita S, Onoda T, Tanno K, Ohsawa M, et al. Low-grade albuminuria and incidence of cardiovascular disease and all-cause mortality in nondiabetic and normotensive individuals. J Hypertens. 2016;34: 506–12; discussion 512.
[19]. Currie G, McKay G, Delles C. Biomarkers in diabetic nephropathy: Present and future. World J Diabetes. 2014;5: 763–776.
[20]. Kim J-J, Hwang B-H, Choi IJ, Choo E-H, Lim S, Koh Y-S, et al. A prospective two-center study on the associations between microalbuminuria, coronary atherosclerosis and long-term clinical outcome in asymptomatic patients with type 2 diabetes mellitus: evaluation by coronary CT angiography. Int J Cardiovasc Imaging. 2015;31: 193–203.
[21]. Kasapoglu B, Turkay C, Yalcın KS, Boga S, Bozkurt A. Increased microalbuminuria prevalence among patients with nonalcoholic fatty liver disease. Ren Fail. 2016;38: 15–19.
[22]. Kim H, Kim HJ, Shin N, Han M, Park H, Kim M, et al. Visceral obesity is associated with microalbuminuria in nondiabetic Asians. Hypertens Res. 2014;37: 679–684.
[23]. Amorim R, Soares P, Chavarria D, Benfeito S, Cagide F, Teixeira J, et al. Decreasing the burden of non-alcoholic fatty liver disease: From therapeutic targets to drug discovery opportunities. Eur J Med Chem. 2024;277: 116723.
[24]. Balamithra S, Devi RG, Jyothipriya A. Estimation of gamma-glutamyl transferase level in liver cancer patients. Drug Invention Today. 2019;12: 1781–1783.
[25]. Mahmoud HE-DA, Yousry WA, Saleh SA, El Badry M, Hussein A, Ali MH, et al. Renal resistive index in non-alcoholic fatty liver disease as an indicator of early renal affection. Egypt Liver J. 2020;10. doi:10.1186/s43066-019-0006-7.
[26]. Wang Y, Fang Y. Postabsorptive homeostasis model assessment for insulin resistance is a reliable biomarker for cardiovascular disease mortality and all-cause mortality. Diabet Epidemiol Manag. 2022;6: 100045.
[27]. Lingli X, Qing Z, Wenfang X. Diagnostic value of the Modification of Diet in Renal Disease and Chronic Kidney Disease Epidemiology Collaboration equations in diabetic patients: a systematic review and meta-analysis. J Int Med Res. 2020;48: 300060520925950.
[28]. Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. BMJ. 1986;292: 13–15.
[29]. Perdomo CM, Garcia-Fernandez N, Escalada J. Diabetic kidney disease, cardiovascular disease and non-alcoholic fatty liver disease: A new triumvirate? J Clin Med. 2021;10: 2040.
[30]. Carulli L, Lonardo A, Lombardini S, Marchesini G, Loria P. Gender, fatty liver and GGT. Hepatology (Baltimore, Md.). Ovid Technologies (Wolters Kluwer Health); 2006. pp. 278–279.
[31]. El Azeem HA, Khalek E-SA, El-Akabawy H, Naeim H, Khalik HA, Alfifi AA. Association between nonalcoholic fatty liver disease and the incidence of cardiovascular and renal events. J Saudi Heart Assoc. 2013;25: 239–246.
[32]. Hwang ST, Cho YK, Yun JW, Park JH, Kim HJ, Park DI, et al. Impact of non-alcoholic fatty liver disease on microalbuminuria in patients with prediabetes and diabetes. Intern Med J. 2010;40: 437–442.
[33]. Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis. 2014;64: 638–652.
[34]. Mantovani A, Zaza G, Byrne CD, Lonardo A, Zoppini G, Bonora E, et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism. 2017;79: 64–76.
Viewed PDF 190 5 -
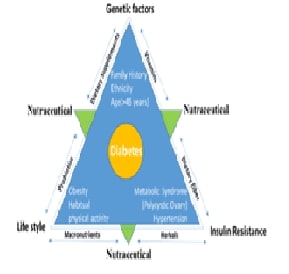 The Complexity of Diabetes Mellitus: Pathophysiology, Prevalence, and Innovative Management StrategiesAuthor: Prabhudas NelaturiDOI: 10.21522/TIJPH.2013.SE.24.05.Art034
The Complexity of Diabetes Mellitus: Pathophysiology, Prevalence, and Innovative Management StrategiesAuthor: Prabhudas NelaturiDOI: 10.21522/TIJPH.2013.SE.24.05.Art034The Complexity of Diabetes Mellitus: Pathophysiology, Prevalence, and Innovative Management Strategies
Abstract:
In recent years, the global use of nutraceuticals, nutritional supplements, and natural therapies for therapeutic purposes has grown significantly. Traditional synthetic drugs often fall short in addressing the therapeutic needs of managing diabetes. In contrast, herbal medicines offer a promising alternative with fewer side effects. Nutraceuticals include a wide range of biological interventions such as amino acids, fatty acids, botanicals, antioxidants, vitamins, and minerals designed to enhance overall well-being, manage symptoms, and prevent diseases. These agents offer diverse therapeutic benefits and show potential in disease management, prevention, and health promotion. Numerous nutraceuticals in clinical practice address the root causes of diabetes mellitus, metabolic syndrome, and their related complications, thereby positively influencing various biochemical and clinical outcomes. Hypoglycemic agents are commonly used in traditional medicine systems to mitigate the risk of diabetes mellitus. In this review, we highlight the risk factors and regional approaches to utilizing nutraceuticals for diabetes management.
The Complexity of Diabetes Mellitus: Pathophysiology, Prevalence, and Innovative Management Strategies
References:
[1]. Forouhi NG, & Wareham NJ. 2019, Epidemiology of diabetes. Medicine, 47(1), 22-7.
[2]. Wild S, Roglic G, Green A, Sicree R, & King H. 2004, Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care, 27(5), 1047-53.
[3]. Rathmann W, & Giani G. 2004, Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030: Response to Wild et al. Diabetes Care, 27(10), 2568-9.
[4]. Chawla A, Chawla R, & Jaggi S. 2016, Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian Journal of Endocrinology and Metabolism, 20(4), 546.
[5]. Association AD. 2014, Diagnosis and classification of diabetes mellitus. Diabetes Care,37(Supplement_1), S81-S90.
[6]. O’Donnell C. 34 - Diabetes. In: Efron N, editor. Contact Lens Practice (Third Edition). Elsevier; 2018. p. 314-20.e1.
[7]. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, & Uribe KB, et al. 2020, Pathophysiology of type 2 diabetes mellitus. International Journal of Molecular Sciences, 21(17), 6275.
[8]. Santini A, Cammarata SM, Capone G, Ianaro A, Tenore GC, & Pani L, et al. 2018, Nutraceuticals: Opening the debate for a regulatory framework. British Journal of Clinical Pharmacology, 84(4), 659-72.
[9]. da Costa JP. 2017, A current look at nutraceuticals – Key concepts and future prospects. Trends in Food Science & Technology, 62, 68-78.
[10]. Vijay VR, I PK, Kumar MB, & Sagetha J. 2024, Assessment of Quality of Life Among Type 2 Diabetes Mellitus Patients in an Urban Health Center of Tiruvallur District, Tamil Nadu. Cureus, 16(6), e63320.
[11]. Redondo MJ, Steck AK, & Pugliese A. 2018, Genetics of type 1 diabetes. Pediatric Diabetes, 19(3), 346-53.
[12]. Burrack AL, Martinov T, & Fife BT. 2017, T cell-mediated beta cell destruction: autoimmunity and alloimmunity in the context of type 1 diabetes. Frontiers in Endocrinology, 8, 343.
[13]. Coppieters KT, Boettler T, & von Herrath M. 2012, Virus infections in type 1 diabetes. Cold Spring Harbor Perspectives in Medicine, 2(1), a007682.
[14]. Yadalam PK, Arumuganainar D, Ronsivalle V, Di Blasio M, Badnjevic A, & Marrapodi MM, et al. 2024, Prediction of interactomic hub genes in PBMC cells in type 2 diabetes mellitus, dyslipidemia, and periodontitis. BMC Oral Health, 24(1), 385.
[15]. Varalakshmi D, Rekha K, & Mohammed R. 2024, Type 2 Diabetes Mellitus Prevalence and Associated Risk Factors in Postmenopausal Women. Cureus, 16(5), e60247.
[16]. Lancet T, & 2023, Diabetes: a defining disease of the 21st century. p. 2087.
[17]. Pinkney J, Tomlinson J, & Stenhouse E. Chapter 2 - Overview of Type 2 Diabetes. In: Bagchi D, Sreejayan N, editors. Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome. San Diego: Academic Press; 2012. p. 15-27.
[18]. Lewandowska M, Więckowska B, & Sajdak S. 2020, Pre-pregnancy obesity, excessive gestational weight gain, and the risk of pregnancy-induced hypertension and gestational diabetes mellitus. Journal of Clinical Medicine, 9(6), 1980.
[19]. Hedderson MM, Darbinian JA, & Ferrara A. 2010, Disparities in the risk of gestational diabetes by race‐ethnicity and country of birth. Paediatric and Perinatal Epidemiology, 24(5), 441-8.
[20]. Kalra EK. 2003, Nutraceutical-definition and introduction. Aaps Pharmsci, 5(3), 25.
[21]. Nasri H, Baradaran A, Shirzad H, & Rafieian-Kopaei M. 2014, New concepts in nutraceuticals as alternative for pharmaceuticals. International Journal of Preventive Medicine,5(12),1487.
[22]. Aronson JK. 2017, Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. British Journal of Clinical Pharmacology, 83(1), 8-19.
[23]. Mikolaskova I, Crnogorac-Jurcevic T, Smolkova B, & Hunakova L. 2023, Nutraceuticals as Supportive Therapeutic Agents in Diabetes and Pancreatic Ductal Adenocarcinoma: A Systematic Review. Biology, 12(2), 158.
[24]. Cencic A, & Chingwaru W. 2010, The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients, 2(6), 611-25.
[25]. Mali S, Rathod S, Kale N, & Shinde N. 2022, Overview of nutraceuticals. Asian Journal of Pharmaceutical Research,12(1),61-70.
[26]. Kelsey NA, Wilkins HM, & Linseman DA. 2010, Nutraceutical antioxidants as novel neuroprotective agents. Molecules, 15(11), 7792-814.
[27]. Rysz J, Franczyk B, Kujawski K, Sacewicz-Hofman I, Ciałkowska-Rysz A, & Gluba-Brzózka A. 2021, Are nutraceuticals beneficial in chronic kidney disease? Pharmaceutics, 13(2), 231.
[28]. Abdelrahman KM, & Hackshaw KV. 2021, Nutritional supplements for the treatment of neuropathic pain. Biomedicines, 9(6), 674.
[29]. Sosnowska B, Penson P, & Banach M. 2017, The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovascular Diagnosis and Therapy, 7(Suppl 1), S21.
[30]. Bertuccioli A, Cardinali M, Biagi M, Moricoli S, Morganti I, & Zonzini GB, et al. 2021, Nutraceuticals and Herbal Food Supplements for Weight Loss: Is There a Prebiotic Role in the Mechanism of Action? Microorganisms, 9(12), 2427.
[31]. Tripathi C, Girme A, Champaneri S, Patel RJ, & Hingorani L. 2020, Nutraceutical regulations: An opportunity in ASEAN countries. Nutrition, 74, 110729.
[32]. Basu SK, Thomas JE, & Acharya SN. 2007, Prospects for growth in global nutraceutical and functional food markets: a Canadian perspective. Australian Journal of Basic and Applied Sciences, 1(4), 637-49.
[33]. Derosa G, Limas CP, Macías PC, Estrella A, & Maffioli P. 2014, Dietary and nutraceutical approach to type 2 diabetes. Arch Med Sci, 10(2), 336-44.
[34]. Khodavirdipour A, Haddadi F, & Keshavarzi S. 2020, Chromium supplementation; negotiation with diabetes mellitus, hyperlipidemia and depression. Journal of Diabetes & Metabolic Disorders, 19, 585-95.
[35]. Sales CH, & Pedrosa LdFC. 2006, Magnesium and diabetes mellitus: Their relation. Clinical Nutrition, 25(4), 554-62.
[36]. Barbagallo M, & Dominguez LJ. 2015, Magnesium and type 2 diabetes. World J Diabetes,6(10),1152-7.
[37]. Pessoa JC, Etcheverry S, & Gambino D. 2015, Vanadium compounds in medicine. Coordination Chemistry Reviews, 301, 24-48.
[38]. Jain AB, & Jain VA. 2012, Vitamin E, its beneficial role in diabetes mellitus (DM) and its complications. Journal of Clinical and Diagnostic Research: JCDR, 6(10), 1624.
[39]. Hajhashemy Z, Rouhani P, & Saneei P. 2022, Dietary calcium intake in relation to type-2 diabetes and hyperglycemia in adults: a systematic review and dose–response meta-analysis of epidemiologic studies. Scientific Reports, 12(1), 1050.
[40]. Thenmozhi P, Sekar R, Bhuvaneswari G, & Tamilselvi S. 2024, Vitamin D supplementation of glycemic control in type 2 diabetes mellitus patients: a randomized controlled study. International Journal of Nutrition, Pharmacology, Neurological Diseases, 14(3).
[41]. Mason SA, Keske MA, & Wadley GD. 2021, Effects of vitamin C supplementation on glycemic control and cardiovascular risk factors in people with type 2 diabetes: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Diabetes Care, 44(2), 618-30.
[42]. Elbadawy AM, Abd Elmoniem RO, & Elsayed AM. 2021, Alpha lipoic acid and diabetes mellitus: potential effects on peripheral neuropathy and different metabolic parameters. Alexandria Journal of Medicine, 57(1), 113-20.
[43]. Golbidi S, Badran M, & Laher I. 2011, Diabetes and alpha lipoic acid. Frontiers in Pharmacology, 2, 69.
[44]. Shen Q, Pierce JD, & 2015, Supplementation of coenzyme Q10 among patients with type 2 diabetes mellitus. MDPI. p. 296-309.
[45]. Testai L, Martelli A, Flori L, Cicero AF, & Colletti A. 2021, Coenzyme Q10: clinical applications beyond cardiovascular diseases. Nutrients, 13(5), 1697.
[46]. Chauhan S, Kodali H, Noor J, Ramteke K, & Gawai V. 2017, Role of omega-3 fatty acids on lipid profile in diabetic dyslipidaemia: single blind, randomised clinical trial. Journal of Clinical and Diagnostic Research: JCDR, 11(3), OC13.
[47]. Mingrone G. 2004, Carnitine in type 2 diabetes. Ann N Y Acad Sci, 1033, 99-107.
[48]. Virmani MA, & Cirulli M. 2022, The role of l-carnitine in mitochondria, prevention of metabolic inflexibility and disease initiation. International Journal of Molecular Sciences, 23(5), 2717.
[49]. Rajasekar P, & Anuradha CV. 2007, Effect of L-carnitine on skeletal muscle lipids and oxidative stress in rats fed high-fructose diet. Exp Diabetes Res, 2007, 72741.
[50]. Modak M, Dixit P, Londhe J, Ghaskadbi S, & Devasagayam TPA. 2007, Indian herbs and herbal drugs used for the treatment of diabetes. Journal of Clinical Biochemistry and Nutrition, 40(3), 163-73.
[51]. Mishra AP, Srivastav N, Singh A, Nigam M, Pezzani R, & Egbuna C, et al. 2022, The Role of Nutraceuticals as Food and Medicine, Types and Sources. In: Egbuna C, Sawicka B, Khan J, editors. Food and Agricultural Byproducts as Important Source of Valuable Nutraceuticals. Cham: Springer International Publishing, 1-18.
[52]. Das L, Bhaumik E, Raychaudhuri U, & Chakraborty R. 2012, Role of nutraceuticals in human health. Journal of Food Science and Technology, 49, 173-83.
Viewed PDF 188 4 -
 Charting the Monthly Waves: An Observational Study on the Dynamics of Premenstrual SyndromeAuthor: Akshaya RadhakrishnanDOI: 10.21522/TIJPH.2013.SE.24.05.Art035
Charting the Monthly Waves: An Observational Study on the Dynamics of Premenstrual SyndromeAuthor: Akshaya RadhakrishnanDOI: 10.21522/TIJPH.2013.SE.24.05.Art035Charting the Monthly Waves: An Observational Study on the Dynamics of Premenstrual Syndrome
Abstract:
Premenstrual syndrome (PMS) is an assortment of painful symptoms that occur around the period of menstruation and they are related to hormonal changes that have been known by physicians for millennia, potentially leading to difficulties in daily functioning and a poor quality of living. The Moos Menstrual Distress Questionnaire (MDQ) was used to gather data in this cross-sectional research with 200 participants in Chennai. ANOVA and T-tests were used to examine the data using SPSS software. Participants were provided with PMS reporting diaries to document symptoms for two consecutive menstrual cycles. The 168 total sample sizes were calculated using the equation of power analysis. There were no irregular menstrual periods among the girls. The average age of the participants was 20.3 years and their average menarche age was 13.5 years. Notably, 124 (73.8%) of individuals reported having considerable blood flow during their menstrual cycle, with 142 (84.5%) of the individuals reported having menstrual cramps. There was no discernible correlation found between the prevalence of PMS and the use concerning junk food, physical activity, sugar, salt, or citrus fruits. Although menstruation is a natural physiological process, many individuals experience menstrual abnormalities and PMS, which require treatment. It is recognized that a significant portion of people have irregular periods and PMS highlights the need for efficient treatment approaches, even though menstruation is a normal physiological function.
Charting the Monthly Waves: An Observational Study on the Dynamics of Premenstrual Syndrome
References:
[1]. Barker-Smith, H., 2020, Navigating the menstrual landscapes: From the darkness to the light. Psychotherapy and Politics International, 18(2), e1533. https://doi.org/10.1002/ppi.1533.
[2]. Hamidovic, A., Karapetyan, K., Serdarevic, F., Choi, S.H., Eisenlohr-Moul, T., & Pinna, G., 2020, Higher circulating cortisol in the follicular vs. luteal phase of the menstrual cycle: A meta-analysis. Frontiers in Endocrinology, 11, 311. https://doi.org/10.3389/fendo.2020.00311.
[3]. Tiranini, L., & Nappi, R.E., 2022, Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Reviews, 11. https://doi.org/10.12703/r/11-9.
[4]. Abbas, K., Usman, G., Ahmed, M., Qazi, R., Asghar, A., Shah, A.M., Rizvi, A., Abid, K., Haq, K.U., Tahir, A., & Usama, S.M., 2020, Physical and psychological symptoms associated with premenstrual syndrome and their impact on the daily routine of women in a low socioeconomic status locality. Cureus, 12(10). https://doi.org/10.7759/cureus.1111.
[5]. Akın, Ö., & Erbil, N., 2023, Investigation of coping behaviors and premenstrual syndrome among university students. Current Psychology, 1-11. https://doi.org/10.1007/s12144-023-03892-5
[6]. Chan, K., Rubtsova, A.A., & Clark, C.J., 2023, Exploring diagnosis and treatment of premenstrual dysphoric disorder in the US healthcare system: A qualitative investigation. BMC Women's Health, 23(1), 1-9. https://doi.org/10.1186/s12905-023-02222-0.
[7]. Batool, S., & Haleem, M., 2022, Unveiling the social studies panorama: Unveiling the interconnectedness of human phenomena. Social Sciences Spectrum, 1(1), 21-29.
[8]. Collatuzzo, G., & Boffetta, P., 2022, Application of P4 (predictive, preventive, personalized, participatory) approach to occupational medicine. La Medicina del Lavoro, 113(1). https://doi.org/10.23749/mdl.v113i1.11939.
[9]. Kamel, D.M., Tantawy, S.A., Alsayed, N., Bekhet, A.H., Elbkery, N., & Khairy, A., 2021, The relationship between premenstrual syndrome and the quality of sleep among Egyptian women: An observational study. Archives of the Balkan Medical Union, 56(2), 172-178.
[10]. Takayama, E., Tanaka, H., Kamimoto, Y., Sugiyama, T., Okano, T., Kondo, E., & Ikeda, T., 2020, Relationship between a high Edinburgh Postnatal Depression Scale score and premenstrual syndrome: A prospective, observational study. Taiwanese Journal of Obstetrics and Gynecology, 59(3), 356-360. https://doi.org/10.1016/j.tjog.2020.03.001.
[11]. Hashim, M.S., Obaideen, A.A., Jahrami, H.A., Radwan, H., Hamad, H.J., Owais, A.A., Alardah, L.G., Qiblawi, S., Al-Yateem, N., & Faris, M.E.A.I.E., 2019, Premenstrual syndrome is associated with dietary and lifestyle behaviors among university students: A cross-sectional study from Sharjah, UAE. Nutrients, 11(8), 1939. https://doi.org/10.3390/nu11081939.
[12]. Helmy, N.A., Kamel, D.M., Gabr, A.A., & Shehata, M.A. Does dietary habits/food consumption affect the premenstrual syndrome incidence among a cohort of Egyptian females: An observational study.
[13]. Rezende, A.P.R., Alvarenga, F.R., Ramos, M., Franken, D.L., Costa, J.S.D.D., Pattussi, M.P., & Paniz, V.M.V., 2022, Prevalence of premenstrual syndrome and associated factors among academics of a university in midwest Brazil. Revista Brasileira de Ginecologia e Obstetrícia, 44, 133-141. https://doi.org/10.1055/s-0041-1739560.
[14]. Heydari, N., Abootalebi, M., Tayebi, N., Hassanzadeh, F., Kasraeian, M., Emamghoreishi, M., & Akbarzadeh, M., 2019, The effect of aromatherapy on mental, physical symptoms, and social functions of females with premenstrual syndrome: A randomized clinical trial. Journal of Family Medicine and Primary Care, 8(9), 2990. https://doi.org/10.4103/jfmpc.jfmpc_472_19.
[15]. Azhary, J.M.K., Leng, L.K., Razali, N., Sulaiman, S., Wahab, A.V.A., Adlan, A.S.A., & Hassan, J., 2022, The prevalence of menstrual disorders and premenstrual syndrome among adolescent girls living in North Borneo, Malaysia: A questionnaire-based study. BMC Women's Health, 22(1), 1-9. https://doi.org/10.1186/s12905-022-01714-7.
[16]. Kim, Y.J., & Park, Y.J., 2020, Menstrual cycle characteristics and premenstrual syndrome prevalence based on the daily record of severity of problems in Korean young adult women. Journal of Korean Academy of Nursing, 50(1), 147-157. https://doi.org/10.4040/jkan.2020.50.1.147.
[17]. Shrestha, D.B., Shrestha, S., Dangol, D., Aryal, B.B., Shrestha, S., Sapkota, B., & Rai, S., 2019, Premenstrual syndrome in students of a teaching hospital. Journal of Nepal Health Research Council, 17(2), 253-257. https://doi.org/10.33314/jnhrc.v17i2.1996.
[18]. Abbas, K., Usman, G., Ahmed, M., Qazi, R., Asghar, A., Shah, A.M., Rizvi, A., Abid, K., Haq, K.U., Tahir, A., & Usama, S.M., 2020, Physical and psychological symptoms associated with premenstrual syndrome and their impact on the daily routine of women in a low socioeconomic status locality. Cureus, 12(10). https://doi.org/10.7759/cureus.11110.
[19]. Thakur, H., Pareek, P., Sayyad, M.G., & Otiv, S., 2022, Association of premenstrual syndrome with adiposity and nutrient intake among young Indian women. International Journal of Women's Health, 665-675. https://doi.org/10.2147/IJWH.S344383.
[20]. Huwaida, D.Z., & Dewi, Y.L.R., 2022, Unhealthy diet and stress are correlated with premenstrual syndrome in adolescent girls in Tangerang. Media Gizi Indonesia, 17(2). https://doi.org/10.20473/mgi.v17i2.61-67.
Viewed PDF 196 3 -
 Control of Multidrug-Resistant Hospitalized Pathogenic Bacteria Using the Secondary Metabolites of Calotropis procera and In-silico Analysis of Bacterial Virulent ProteinsAuthor: Thangaswamy SelvankumarDOI: 10.21522/TIJPH.2013.SE.24.05.Art036
Control of Multidrug-Resistant Hospitalized Pathogenic Bacteria Using the Secondary Metabolites of Calotropis procera and In-silico Analysis of Bacterial Virulent ProteinsAuthor: Thangaswamy SelvankumarDOI: 10.21522/TIJPH.2013.SE.24.05.Art036Control of Multidrug-Resistant Hospitalized Pathogenic Bacteria Using the Secondary Metabolites of Calotropis procera and In-silico Analysis of Bacterial Virulent Proteins
Abstract:
This study explores the multidrug-resistant pattern of hospitalized pathogens and their pharmacological impact against the secondary metabolites isolated from Calotropis procera, for its medicinal properties. Moreover, this study implies that comprehensive analysis, including isolation of multidrug-resistant hospitalized bacterial species and extraction and characterization of secondary metabolites by GC-MS from Calotropis procera, molecular docking, ADMET profiling, and, was conducted to evaluate the therapeutic potential of these compounds. The multidrug-resistant Klebsiella pneumonia, Salmonella typhi, and Pseudomonas aeruginosa were isolated and they also showed sensitivity against C. procera leaf extract. GC-MS reveals the key volatile compounds, including oleic acid, and the molecular docking with the proteasome (PDB ID: 5JXG) identified 5-Methyl-2-phenylindolizine as the most promising compound due to its high binding affinity (-6.7 kcal/mol), with 2,6-Dimethylphenol, 3,5-Dimethylaniline, and Ethyl Heptanoate showing progressively lower affinities. Interaction analysis highlighted the importance of PRO266, TRP531, GLU271, and ARG490 residues. ADMET profiling revealed that 2,6-dimethylphenol and 3,5-Dimethylaniline have favorable absorption and minimal CYP interactions, while 5-methyl-2-phenylindolizine demonstrated excellent absorption and BBB permeability. Additionally, the study found that C. procera metabolites could target furin, a proprotein convertase involved in bacterial virulence, offering a novel approach to combat multidrug-resistant bacterial infections. These findings underscore the potential of Calotropis procera metabolites as effective therapeutic agents and active against multidrug-resistant bacterial species.
Control of Multidrug-Resistant Hospitalized Pathogenic Bacteria Using the Secondary Metabolites of Calotropis procera and In-silico Analysis of Bacterial Virulent Proteins
References:
[1]. Salam MA, Al-Amin MY, Salam MT, Pawar JS, Akhter N, Rabaan AA, et al. 2023., Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare.11: 1946. doi:10.3390/healthcare11131946
[2]. Wang J, Xu S, Zhao K, Song G, Zhao S, Liu R., 2023, Risk control of antibiotics, antibiotic resistance genes (ARGs) and antibiotic-resistant bacteria (ARB) during sewage sludge treatment and disposal: A review. Sci Total Environ. 877: 162772. doi:10.1016/j.scitotenv.2023.162772
[3]. Sharma S, Chauhan A, Ranjan A, Mathkor DM, Haque S, Ramniwas S, et al., 2024, Emerging challenges in antimicrobial resistance: implications for pathogenic microorganisms, novel antibiotics, and their impact on sustainability. Front Microbiol.15. doi:10.3389/fmicb.2024.1403168
[4]. Ahmed SK, Hussein S, Qurbani K, Ibrahim RH, Fareeq A, Mahmood KA, et al., 2024, Antimicrobial resistance: Impacts, challenges, and prospects. J Med Surgery, Public Heal. 2: 100081. doi:10.1016/j.glmedi.2024.100081
[5]. Angelini P., 2024, Plant-Derived Antimicrobials and Their Crucial Role in Combating Antimicrobial Resistance. Antibiotics.;13: 746. doi:10.3390/antibiotics13080746
[6]. Salam U, Ullah S, Tang Z-H, Elateeq AA, Khan Y, Khan J, et al., 2023, Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life.;13: 706. doi:10.3390/life13030706
[7]. Divekar PA, Narayana S, Divekar BA, Kumar R, Gadratagi BG, Ray A, et al., 2022, Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int J Mol Sci. 23: 2690. doi:10.3390/ijms23052690
[8]. Sorescu A-A, Nuta A, Ion R-M, Iancu L., 2018, Qualitative Analysis of Phytochemicals from Sea Buckthorn and Gooseberry. Phytochemicals - Source of Antioxidants and Role in Disease Prevention. InTech; doi:10.5772/intechopen.77365
[9]. Ku Y-S, Contador CA, Ng M-S, Yu J, Chung G, Lam H-M., 2020, The Effects of Domestication on Secondary Metabolite Composition in Legumes. Front Genet.11. doi:10.3389/fgene.2020.581357
[10]. Abeed AHA, Ali M, Ali EF., 2021, Majrashi A, Eissa MA. Induction of Catharanthus roseus Secondary Metabolites When Calotropis procera Was Used as Bio-Stimulant. Plants.;10: 1623. doi:10.3390/plants10081623
[11]. Wadhwani BD, Mali D, Vyas P, Nair R, Khandelwal P., 2021, A review on phytochemical constituents and pharmacological potential of Calotropis procera. RSC Adv. 11: 35854–35878. doi:10.1039/D1RA06703F
[12]. Habeeb A, Ramesh S, Shanmugam R., 2024, Calotropis procera and the Pharmacological Properties of Its Aqueous Leaf Extract: A Review. Cureus. doi:10.7759/cureus.60354
[13]. Ong CY, Ling SK, Ali RM, Chee CF, Samah ZA, Ho ASH, et al., 2009, Systematic analysis of in vitro photo-cytotoxic activity in extracts from terrestrial plants in Peninsula Malaysia for photodynamic therapy. J Photochem Photobiol B Biol. 96: 216–222. doi:10.1016/j.jphotobiol.2009.06.009
[14]. Yang Y, Liang X, Jin P, Li N, Zhang Q, Yan W, et al., 2019, Screening and determination for potential acetylcholinesterase inhibitory constituents from ginseng stem–leaf saponins using ultrafiltration (UF)-LC-ESI-MS2. Phytochem Anal. 30: 26–33. doi:10.1002/pca.2787
[15]. Ito A, Lai CH, Zhao X, Saito S, Hamilton MH, Appella E, et al., 2001,p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 20: 1331–1340. doi:10.1093/emboj/20.6.1331
[16]. Rahiman FA, Mahmad N, Taha RM, Elias H, Zaman FH., 2013, Antimicrobial properties of Lawsonia inermis syn. Lawsonia alba in vivo and in vitro. J Food, Agric Environ. 11: 502–504.
[17]. Padmini R, Uma Maheshwari V, Saravanan P, Woo Lee K, Razia M, Alwahibi MS, et al., 2020, Identification of novel bioactive molecules from garlic bulbs: A special effort to determine the anticancer potential against lung cancer with targeted drugs. Saudi J Biol Sci. 27: 3274–3289. doi:10.1016/j.sjbs.2020.09.041
[18]. Kirubhanand C, Merciline Leonora J, Anitha S, Sangeetha R, Nachammai KT, Langeswaran K, et al., 2023, Targeting potential receptor molecules in non-small cell lung cancer (NSCLC) using in silico approaches. Front Mol Biosci. 10: 1–16. doi:10.3389/fmolb.2023.1124563
[19]. Braun E, Sauter D., 2019, Furin‐mediated protein processing in infectious diseases and cancer. Clin Transl Immunol. 8. doi:10.1002/cti2.1073
[20]. Douglas LEJ, Reihill JA, Montgomery BM, Martin SL., 2023, Furin as a therapeutic target in cystic fibrosis airways disease. Eur Respir Rev. 32: 220256. doi:10.1183/16000617.0256-2022
[21]. Krueger E, Brown AC., 2019, Inhibition of bacterial toxin recognition of membrane components as an anti-virulence strategy. J Biol Eng. 13: 4. doi:10.1186/s13036-018-0138-z
[22]. do Vale A, Cabanes D, Sousa S., 2016, Bacterial Toxins as Pathogen Weapons Against Phagocytes. Front Microbiol. 7. doi:10.3389/fmicb.2016.00042
[23]. Zhang H, Zhang Z, Li J, Qin G., 2023, New Strategies for Biocontrol of Bacterial Toxins and Virulence: Focusing on Quorum-Sensing Interference and Biofilm Inhibition. Toxins (Basel). 15: 570. doi:10.3390/toxins15090570
[24]. Devi KP, Pourkarim MR, Thijssen M, Sureda A, Khayatkashani M, Cismaru CA, et al., 2022, A perspective on the applications of furin inhibitors for the treatment of SARS-CoV-2. Pharmacol Reports. 74: 425–430. doi:10.1007/s43440-021-00344-x
[25]. Jaganathan R, Kumaradhas P., 2024, Structural insights into Furin enzyme inhibition to block SARS-CoV-2 spike protein cleavage: an in-silico approach. 3 Biotech. 14: 213. doi:10.1007/s13205-024-04054-y
[26]. Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al., 2010, AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J Comput Chem. 30: 2785–2791. doi:10.1002/jcc.21256.AutoDock4
[27]. Berman HM, Battistuz T, Bhat TN, Bluhm WF, Bourne PE, Burkhardt K, et al., 2002, The protein data bank. Acta Crystallogr Sect D Biol Crystallogr. 58: 899–907. doi:10.1107/S0907444902003451
[28]. Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. 2021, PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 49, D1388–D1395. doi:10.1093/nar/gkaa971
[29]. Dahms SO, Arciniega M, Steinmetzer T, Huber R, Than ME., 2016, Structure of the unliganded form of the proprotein convertase furin suggests activation by a substrate-induced mechanism. Proc Natl Acad Sci U S A. 113: 11196–11201. doi:10.1073/pnas.1613630113
[30]. DeLano WL., 2002, Pymol: An open-source molecular graphics tool. CCP4 Newsl Protein Crystallogr. 40: 82–92.
[31]. BIOVIA. 2016. Discovery Studio Modeling Environment. San Diego: Dassault Systems.
[32]. Pires DE V., Blundell TL, Ascher DB., 2015, pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J Med Chem. 58: 4066–4072. doi:10.1021/acs.jmedchem.5b00104
[33]. Fiehn O., 2016, Metabolomics by Gas Chromatography–Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr Protoc Mol Biol. 114. doi:10.1002/0471142727.mb3004s114
[34]. Jeyasundari J, Praba P, Jacob Y, Rajendran S, Kaleeswari K., 2016, Green Synthesis and Characterization of Silver Nanoparticles Using Mimusops elengi Flower Extract and Its Synergistic Antimicrobial Potential. Am Chem Sci J. 12: 1–11. doi:10.9734/ACSJ/2016/23161
[35]. Deshmukh SP, Patil SM, Mullani SB, Delekar SD., 2019, Silver nanoparticles as an effective disinfectant: A review. Mater Sci Eng C. 97: 954–965. doi:10.1016/j.msec.2018.12.102
Viewed PDF 200 9 -
 Green Synthesis of Silver Nanoparticles Using Coriandrum sativum Extract and Their Antioxidant, Anti-inflammatory and Antibacterial Activities Against UTI PathogensAuthor: Lakshmi ThangaveluDOI: 10.21522/TIJPH.2013.SE.24.05.Art037
Green Synthesis of Silver Nanoparticles Using Coriandrum sativum Extract and Their Antioxidant, Anti-inflammatory and Antibacterial Activities Against UTI PathogensAuthor: Lakshmi ThangaveluDOI: 10.21522/TIJPH.2013.SE.24.05.Art037Green Synthesis of Silver Nanoparticles Using Coriandrum sativum Extract and Their Antioxidant, Anti-inflammatory and Antibacterial Activities Against UTI Pathogens
Abstract:
This study explored the green synthesis of silver nanoparticles (AgNPs) utilizing Coriandrum sativum extract and investigated their potential applications in terms of antibacterial and antioxidant activities. UV‒visible absorption spectra revealed a distinctive absorption peak at 450 nm, indicating successful AgNPs synthesis and stabilization with Coriandrum sativum extract and the TEM analysis revealed the shape to be spherical and size was found to range between 5-25 nm. Antibacterial assays revealed concentration-dependent zones of inhibition against Staphylococcus aureus, Escherichia coli, Pseudomonas sp., and Klebsiella sp., confirming the efficacy of Coriandrum sativum-mediated AgNPs as antibacterial agents. The AgNPs exhibited significant antioxidant properties, with concentration-dependent scavenging activities observed in the DPPH, hydrogen peroxide, and FRAP assays. Protein denaturation assays revealed concentration-dependent effects, suggesting potential applications in biological and medicinal contexts. Additionally, membrane stabilization assays revealed the robust concentration-dependent impact of Coriandrum sativum-mediated AgNPs, underscoring their promising role in diverse applications, from antibacterial treatments to antioxidant therapies and protein stability studies.
Green Synthesis of Silver Nanoparticles Using Coriandrum sativum Extract and Their Antioxidant, Anti-inflammatory and Antibacterial Activities Against UTI Pathogens
References:
[1]. Mancuso G, Midiri A, Gerace E, Marra M, Zummo S, Biondo C. 2023, Urinary tract infections: The current scenario and future prospects. Pathogens.12: 623. doi:10.3390/pathogens12040623
[2]. England NHS. NHS England » New awareness campaign to help reduce hospital admissions for urinary tract infections. [cited 11 Mar 2024]. Available: https://www.england.nhs.uk/2023/10/new-awareness-campaign-to-help-reduce-hospital-admissions-for-urinary-tract-infections
[3]. Yang X, Chen H, Zheng Y, Qu S, Wang H, Yi F. 2022, Disease burden and long-term trends of urinary tract infections: A worldwide report. Front Public Health. 10. doi:10.3389/fpubh.2022.888205
[4]. Paul R. 2018, State of the globe: Rising antimicrobial resistance of pathogens in urinary tract infection. J Glob Infect Dis. 10: 117–118. doi:10.4103/jgid.jgid_104_17
[5]. Sánchez SV, Navarro N, Catalán-Figueroa J, Morales JO. 2021, Nanoparticles as potential novel therapies for urinary tract infections. Front Cell Infect Microbiol.11. doi:10.3389/fcimb.2021.656496
[6]. Qindeel M, Barani M, Rahdar A, Arshad R, Cucchiarini M. 2021, Nanomaterials for the diagnosis and treatment of urinary tract infections. Nanomaterials (Basel). 211: 546. doi:10.3390/nano11020546
[7]. Yin IX, Zhang J, Zhao IS, Mei ML, Li Q, Chu CH. 2020, The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J Nanomedicine.15: 2555–2562. doi:10.2147/ijn.s246764
[8]. Divya M, Kiran GS, Hassan S, Selvin J. 2019, Biogenic synthesis and effect of silver nanoparticles (AgNPs) to combat catheter-related urinary tract infections. Biocatal Agric Biotechnol. 18: 101037. doi:10.1016/j.bcab.2019.101037
[9]. Alsubki R, Tabassum H, Abudawood M, Rabaan AA, Alsobaie SF, Ansar S. 2021, Green synthesis, characterization, enhanced functionality and biological evaluation of silver nanoparticles based on Coriander sativum. Saudi J Biol Sci. 2021;28: 2102–2108. doi:10.1016/j.sjbs.2020.12.055
[10]. Rajeshkumar S, Tharani M, Jeevitha M, Santhoshkumar J. 2019, Anticariogenic activity of fresh aloe Vera gel mediated copper oxide nanoparticles. Indian J Public Health Res Dev. 10: 3664. doi:10.5958/0976-5506.2019.04158.5
[11]. Sobhani Z, Mohtashami L, Amiri MS, Ramezani M, Emami SA, Simal-Gandara J. 2022, Ethnobotanical and phytochemical aspects of the edible herb Coriandrum sativum L. J Food Sci. 87: 1386–1422. doi:10.1111/1750-3841.16085
[12]. Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V. 2018, Coriander (Coriandrum sativum): A promising functional food toward the well-being. Food Res Int. 105: 305–323. doi:10.1016/j.foodres.2017.11.019
[13]. Salem MA, Manaa EG, Osama N, Aborehab NM, Ragab MF, Haggag YA, et al. 2022, Coriander (Coriandrum sativum L.) essential oil and oil-loaded nanoformulations as an anti-aging potentiality via TGFβ/SMAD pathway. Sci Rep. 12: 1–15. doi:10.1038/s41598-022-10494-4
[14]. Al-Marzoqi AH, Hameed IH, Idan SA. 2015, Analysis of bioactive chemical components of two medicinal plants (Coriandrum sativum and Melia azedarach) leaves using gas chromatography-mass spectrometry (GC-MS). African Journal of Biotechnology. Oct 23;14(40):2812-30.doi: 10.5897/AJB2015.14956
[15]. Sharma SK, Singh AP. 2012, In vitro antioxidant and free radical scavenging activity of Nardostachys jatamansi DC. J Acupunct Meridian Stud. 5: 112–118. doi:10.1016/j.jams.2012.03.002
[16]. Rajeshkumar S, Malarkodi C, Vanaja M, Annadurai G. 2016, Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. Journal of molecular structure. 15;1116:165-73.
[17]. Bhatti MZ, Ali A, Ahmad A, Saeed A, Malik SA. 2015, Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC Res Notes. 8: 279. doi:10.1186/s13104-015-1228-3
[18]. Devadharshini R, Karpagam G, Pavithra K, Kowsalya S, Priya PM, Ramachandran AM. 2023, Green synthesis of silver nanoparticles. Microbiol Res J Int. 33: 1–9. doi:10.9734/mrji/2023/v33i51380
[19]. Tharani M, Rajeshkumar S, Al-Ghanim KA, Nicoletti M, Sachivkina N, Govindarajan M. 2023, Terminalia chebula-Assisted Silver Nanoparticles: Biological Potential, Synthesis, Characterization, and Ecotoxicity. Biomedicines. 11. doi:10.3390/biomedicines11051472
[20]. Qaeed MA. 2023, Review of Biological methods of AgNPs Synthesis. Journal of Chemistry and Nutritional Biochemistry. 4. doi:10.48185/jcnb.v4i1.793
[21]. Guzmán K, Kumar B, Grijalva M, Debut A, Cumbal L. 2022, Ascorbic acid-assisted green synthesis of silver nanoparticles: pH and stability study. Green Chemistry - New Perspectives. IntechOpen; doi:10.5772/intechopen.107202
[22]. Chinnathambi A, Alharbi SA, Joshi D, Saranya, Jhanani GK, On-uma R, et al. 2023, Synthesis of AgNPs from leaf extract of Naringi crenulata and evaluation of its antibacterial activity against multidrug resistant bacteria. Environ Res. 216: 114455. doi:10.1016/j.envres.2022.114455
[23]. Choudhary S, Kumawat G, Khandelwal M, Khangarot RK, Saharan V, Nigam S, Harish. 2024, Phyco-synthesis of silver nanoparticles by environmentally safe approach and their applications. Scientific Reports. 14(1):9568.doi.org/10.1038/s41598-024-60195-3
[24]. Soleimani M, Habibi-Pirkoohi M. 2017, Biosynthesis of silver nanoparticles using Chlorella vulgaris and evaluation of the antibacterial efficacy against Staphylococcus aureus. Avicenna journal of medical biotechnology. 9(3):120. PMCID: PMC5501138.
[25]. Antioxidant and antibacterial activities of Allium sativum ethanol extract and silver nanoparticles. 2023, Tropical Journal of Natural Product Research. 7. doi:10.26538/tjnpr/v7i6.5
[26]. Ebrahimzadeh MA, Barani A, Habibian AH, Goli HR, Alizadeh SR. 2023, Overcoming multidrug-resistant bacteria and fungi by green synthesis of AgNPs using Nepeta pogonosperma extract, optimization, characterization and evaluation of antibacterial and antifungal effects. Eur J Chem. 14: 254–263. doi:10.5155/eurjchem.14.2.254-263.2404
[27]. Asgarpanah J, Kazemivash N. 2012, Phytochemistry, pharmacology and medicinal properties of Coriandrum sativum L. African Journal of Pharmacy and Pharmacology. 22;6(31):2340-5. doi:10.5897/AJPP12.901
[28]. Ali Ahmed S, Mahmood Hasan H, Ghassan Sweedan E. 2023, Antibacterial action of AgNPs produced from different isolates of gram-positive and gram-negative bacteria on biofilm of Klebsiella pneumoniae isolated from RTI. Biomed (Trivandrum). 43: 983–987. doi:10.51248/.v43i3.2813
[29]. Mudhafar M, Alsailawi HA, Zorah M, Karhib MM, Zainol I, Kadhim FK. Biogenic Synthesis and Characterization of AgNPs Using CEPS: Cytotoxicity and Antibacterial Activites. 2023, Adv Res Fluid Mech Therm Sci. 106: 65–75. doi:10.37934/arfmts.106.1.6575
[30]. Fereydani M, Larijani K, Aziziyan F. 2023, Green synthesis of silver nanoparticles from Polygonum aviculare L. extract, evaluation of antibacterial, antioxidant activity. doi:10.22541/au.168735783.36916760/v1
[31]. Dua TK, Giri S, Nandi G, Sahu R, Shaw TK, Paul P. 2023, Green synthesis of silver nanoparticles using Eupatorium adenophorum leaf extract: characterizations, antioxidant, antibacterial and photocatalytic activities. Chem Pap. 77: 2947–2956. doi:10.1007/s11696-023-02676-9
[32]. Lakkim V, Reddy MC, Lekkala VVV, Lebaka VR, Korivi M, Lomada D. 2023, Antioxidant efficacy of green-synthesized silver nanoparticles promotes wound healing in mice. Pharmaceutics. 15: 1517. doi:10.3390/pharmaceutics15051517
[33]. Baran MF, Keskin C, Baran A, Hatipoğlu A, Yildiztekin M, Küçükaydin S, et al. 2023, Green synthesis of silver nanoparticles from Allium cepa L. peel extract, their antioxidant, antipathogenic, and anticholinesterase activity. Molecules. 28: 2310. doi:10.3390/molecules28052310
[34]. Kurup M, Kumar M, Ramanathan S, Chandra M. 2023, The biogenetic synthesis of metallic nanoparticles and the role they play in anti-inflammatory drug treatment. Curr Drug Discov Technol. 20. doi:10.2174/1570163820666230718123544
[35]. Singh S, Sharma K, Sharma H. 2024, Green extracts with metal-based nanoparticles for treating inflammatory diseases: A review. Curr Drug Deliv. 21: 544–570. doi:10.2174/1567201820666230602164325
[36]. Sharma A, Sanjay, Jaiswal V, Park M, Lee H-J. 2023, Biogenic silver NPs alleviate LPS-induced neuroinflammation in a human fetal brain-derived cell line: Molecular switch to the M2 phenotype, modulation of TLR4/MyD88 and Nrf2/HO-1 signalling pathways, and molecular docking analysis. Biomater Adv. 148: 213363. doi:10.1016/j.bioadv.2023.213363
[37]. Xu Z, Zha X, Ji R, Zhao H, Zhou S. 2023, Green biosynthesis of silver nanoparticles using aqueous extracts of Ageratum conyzoides and their anti-inflammatory effects. ACS Appl Mater Interfaces. doi:10.1021/acsami.2c22114
Viewed PDF 219 15 -
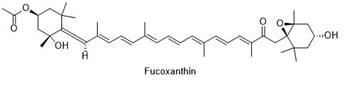 Promising Health Benefits of FucoxanthinAuthor: Palukuri Yashwanth KumarDOI: 10.21522/TIJPH.2013.SE.24.05.Art038
Promising Health Benefits of FucoxanthinAuthor: Palukuri Yashwanth KumarDOI: 10.21522/TIJPH.2013.SE.24.05.Art038Promising Health Benefits of Fucoxanthin
Abstract:
Fucoxanthin, a unique carotenoid found in brown seaweed, contains an allenic bond in its structure along with a cyclic core, conjugated double bonds, and functional groups. It plays a crucial role in photosynthesis by absorbing and transferring light energy to chlorophyll a. It also exhibits health benefits, such as improving immunity and gut health and protective activities, including hepatic, neuro, and nephroprotective against various diseases, which makes it a promising pharmaceutical and dietary component for combating infectious disorders. Recent research focuses on the health-promoting properties of fucoxanthin, highlighting its various health promoting mechanisms, aiming to guide future biochemical studies toward developing new supplements utilizing fucoxanthin and its metabolites. This review provides a foundation for future health-promoting investigations focused on developing novel pharmaceutical and dietary supplements targeting fucoxanthin and its various metabolites
Promising Health Benefits of Fucoxanthin
References:
[1]. Pruccoli, L., Balducci, M., Pagliarani, B., and Tarozzi, A., 2024, Antioxidant and Neuroprotective Effects of Fucoxanthin and Its Metabolite Fucoxanthinol: A Comparative In Vitro Study. Current Issues in Molecular Biology, 46(6), 5984-5998. https://doi.org/10.3390/cimb46060357
[2]. López-Ramos, A., González-Ortiz, M., Martínez-Abundis, E., and Pérez-Rubio, K. G., 2023, Effect of Fucoxanthin on Metabolic Syndrome, Insulin Sensitivity, and Insulin Secretion. Journal of Medicinal Food, 26(7), 521-527. https://doi.org/10.1089/jmf.2022.0103
[3]. Mohibbullah, M., Haque, M. N., Sohag, A. A. M., Hossain, M. T., Zahan, M. S., Uddin, M. J., and Choi, J. S., 2022, A systematic review on marine algae-derived fucoxanthin: An update of pharmacological insights. Marine Drugs, 20(5), 279. https://doi.org/10.3390/md20050279
[4]. Mumu, M., Das, A., Emran, T. B., Mitra, S., Islam, F., Roy, A., and Kim, B. 2022, Fucoxanthin: A promising phytochemical on diverse pharmacological targets. Frontiers in Pharmacology, 13, 929442. https://doi.org/10.3389/fphar.2022.929442
[5]. Sayuti, N. H., Muhammad Nawawi, K. N., Goon, J. A., Mokhtar, N. M., Makpol, S., and Tan, J. K., 2023, A Review of the Effects of Fucoxanthin on NAFLD. Nutrients, 15(8), 1954. https://doi.org/10.3390/nu15081954
[6]. Sun, H., Yang, S., Zhao, W., Kong, Q., Zhu, C., Fu, X., and He, Y., 2023, Fucoxanthin from marine microalgae: A promising bioactive compound for industrial production and food application. Critical Reviews in Food Science and Nutrition, 63(26), 7996-8012. https://doi.org/10.1080/10408398.2022.2054932
[7]. Guan, B., Chen, K., Tong, Z., Chen, L., Chen, Q., and Su, J., 2022, Advances in fucoxanthin research for the prevention and treatment of inflammation-related diseases. Nutrients, 14(22), 4768. https://doi.org/10.3390/nu14224768
[8]. Lau, T. Y., and Kwan, H. Y., 2022, Fucoxanthin is a potential therapeutic agent for the treatment of breast cancer. Marine drugs, 20(6), 370. https://doi.org/10.3390/md20060370
[9]. Pajot, A., Hao Huynh, G., Picot, L., Marchal, L., and Nicolau, E., 2022, Fucoxanthin from algae to human, an extraordinary bioresource: Insights and advances in up and downstream processes. Marine drugs, 20(4), 222. https://doi.org/10.3390/md20040222
[10]. Noviendri, D., Fithriani, D., and Hasrini, R. F., 2020, Fucoxanthin, A Xanthophyll from Macro-and Microalgae: Extraction Techniques, Bioactivities and Their Potential Application in Nutra-and Cosmeceutical Industries. In E3S Web of Conferences (Vol. 232, p. 03010). https://doi.org/10.1051/e3sconf/202123203010
[11]. Koch, W., Kukula-Koch, W., Wawruszak, A., Okoń, E., Stępnik, K., Gaweł-Bęben, K., and Calina, D., 2024, Fucoxanthin: From chemical properties and sources to novel anticancer mechanistic insights and synergistic therapeutic opportunities. Current Research in Biotechnology, 100203. https://doi.org/10.1016/j.crbiot.2024.100203
[12]. Jaiswal, J., Srivastav, A. K., Patel, R., and Kumar, U., 2022, Synthesis and physicochemical characterization of rhamnolipid fabricated fucoxanthin loaded bovine serum albumin nanoparticles supported by simulation studies. Journal of the Science of Food and Agriculture, 102(12), 5468-5477. https://doi.org/10.1002/jsfa.11901
[13]. Su, J., Guan, B., Chen, K., Feng, Z., Guo, K., Wang, X., and Chen, Q., 2023, Fucoxanthin attenuates inflammation via interferon regulatory factor 3 (IRF3) to improve sepsis. Journal of Agricultural and Food Chemistry, 71(33), 12497-12510. https://doi.org/10.1021/acs.jafc.3c03247
[14]. Lykov, A., Rachkovskaya, L., Gevorgiz, R., Zheleznova, S., and Poveshchenko, O., 2022, The Influence of Fucoxanthin Immobilized on Porous Aluminum-Silicon Carrier Surface on the Functional Activities of Immunocytes in Mice. KnE Life Sciences, 77-83. https://knepublishing.com/index.php/KnE-Life/article/view/10109
[15]. Han, B., Ma, Y., and Liu, Y., 2022, Fucoxanthin prevents the ovalbumin-induced food allergic response by enhancing the intestinal epithelial barrier and regulating the intestinal flora. Journal of Agricultural and Food Chemistry, 70(33), 10229-10238. https://doi.org/10.1021/acs.jafc.2c04685
[16]. Lee, A. H., Shin, H. Y., Park, J. H., Koo, S. Y., Kim, S. M., and Yang, S. H., 2021, Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-κB and NLRP3 inflammasome activation. Scientific reports, 11(1), 543. https://doi.org/10.1038/s41598-020-80748-6
[17]. Šudomová, M., Shariati, M. A., Echeverría, J., Berindan-Neagoe, I., Nabavi, S. M., and Hassan, S. T., 2019, A microbiological, toxicological, and biochemical study of the effects of fucoxanthin, a marine carotenoid, on Mycobacterium tuberculosis and the enzymes implicated in its cell wall: A link between mycobacterial infection and autoimmune diseases. Marine drugs, 17(11), 641. https://doi.org/10.3390/md17110641
[18]. Li, X., Huang, R., Liu, K., Li, M., Luo, H., Cui, L., and Luo, L., 2021, Fucoxanthin attenuates LPS-induced acute lung injury via inhibition of the TLR4/MyD88 signaling axis. Aging (albany NY), 13(2), 2655. doi: 10.18632/aging.202309
[19]. Tanaka, K., Natsume, C., Azuma, Y., and Fujita, T., 2018, Fucoxanthin suppress GATA-3 positive immuno-responses. In Proceedings for Annual Meeting of The Japanese Pharmacological Society WCP2018 (The 18th World Congress of Basic and Clinical Pharmacology) (pp. PO2-5). Japanese Pharmacological Society. https://doi.org/10.1254/jpssuppl.WCP2018.0_PO2-5-2
[20]. Yang, X., Guo, G., Dang, M., Yan, L., Kang, X., Jia, K., and Ren, H., 2019, Assessment of the therapeutic effects of fucoxanthin by attenuating inflammation in ovalbumin-induced asthma in an experimental animal model. Journal of Environmental Pathology, Toxicology and Oncology, 38(3). DOI: 10.1615/JEnvironPatholToxicolOncol.2019030154
[21]. Su, J., Guan, B., Su, Q., Hu, S., Wu, S., Tong, Z., and Zhou, F. (2023). Fucoxanthin ameliorates sepsis via modulating microbiota by targeting IRF3 activation. International Journal of Molecular Sciences, 24(18), 13803. DOI: 10.1615/JEnvironPatholToxicolOncol.2019030154
[22]. Xiao, H., Zhao, J., Fang, C., Cao, Q., Xing, M., Li, X., and Song, S., 2020, Advances in studies on the pharmacological activities of fucoxanthin. Marine drugs, 18(12), 634. https://doi.org/10.3390/md18120634
[23]. Bernabeu, M., Gharibzahedi, S. M. T., Ganaie, A. A., Macha, M. A., Dar, B. N., Castagnini, J. M., and Barba, F. J., 2023, The potential modulation of gut microbiota and oxidative stress by dietary carotenoid pigments. Critical Reviews in Food Science and Nutrition, 1-19, https://doi.org/10.1080/10408398.2023.2254383
[24]. Sun, X., Zhao, H., Liu, Z., Sun, X., Zhang, D., Wang, S., and Wang, D., 2020, Modulation of gut microbiota by fucoxanthin during alleviation of obesity in high-fat diet-fed mice. Journal of agricultural and food chemistry, 68(18), 5118-5128. https://doi.org/10.1021/acs.jafc.0c01467
[25]. Guo, B., Yang, B., Pang, X., Chen, T., Chen, F., and Cheng, K. W., 2019, Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food & function, 10(9), 5644-5655. https://doi.org/10.1039/C9FO01018A
[26]. Saxena A, 2024, Anticancer Efficacy of Fucoxanthin in Targeting Ovarian Cancer Cells. International Journal of Life Sciences, Biotechnology and Pharma Research (13),6, 2024 DOI: 10.69605/ijlbpr_13.6.21
[27]. Du, H. F., Wu, J. W., Zhu, Y. S., Hua, Z. H., Jin, S. Z., Ji, J. C., and Ding, H. M., 2024, Fucoxanthin Induces Ferroptosis in Cancer Cells via Downregulation of the Nrf2/HO− 1/GPX4 Pathway. Molecules, 29(12), 2832. https://doi.org/10.3390/molecules29122832
[28]. Terasaki, M., Tsuruoka, K., Tanaka, T., Maeda, H., Shibata, M., Miyashita, K., and Hamada, A., 2023, Fucoxanthin inhibits development of sigmoid colorectal cancer in a PDX model with alterations of growth, adhesion, and cell cycle signals. Cancer Genomics & Proteomics, 20(6suppl), 686-705. DOI: https://doi.org/10.21873/cgp.20416
[29]. Ahmed, S. A., Mendonca, P., Elhag, R., and Soliman, K. F., 2022, Anticancer effects of fucoxanthin through cell cycle arrest, apoptosis induction, angiogenesis inhibition, and autophagy modulation. International Journal of Molecular Sciences, 23(24), 16091. https://doi.org/10.3390/ijms232416091
[30]. Ahmed, S. A., Mendonca, P., Messeha, S. S., Oriaku, E. T., and Soliman, K. F., 2023, The Anticancer Effects of Marine Carotenoid Fucoxanthin through Phosphatidylinositol 3-Kinase (PI3K)-AKT Signaling on Triple-Negative Breast Cancer Cells. Molecules, 29(1), 61. https://doi.org/10.3390/molecules29010061
[31]. Zhang, X. Q., Liu, T. Y., Zhang, L. T., Hua, Z. H., Jin, X. A., Xu, F., and Ding, H. M., 2023, Effects and Mechanisms of Fucoxanthin from Hizikia fusiforme on Inhibiting Tongue Squamous Cell Carcinoma Proliferation via AKT/mTOR‐Mediated Glycolysis. Journal of Food Biochemistry, 2023(1), 7944733. https://doi.org/10.1155/2023/7944733
[32]. Luan, H., Yan, L., Zhao, Y., Ding, X., and Cao, L., 2022, Fucoxanthin induces apoptosis and reverses epithelial-mesenchymal transition via inhibiting Wnt/β-catenin pathway in lung adenocarcinoma. Discover Oncology, 13(1), 98. https://doi.org/10.1007/s12672-022-00564-4
[33]. Yang, S., Li, J., Yan, L., Wu, Y., Zhang, L., Li, B., and Lin, X., 2024. Molecular Mechanisms of Fucoxanthin in Alleviating Lipid Deposition in Metabolic Associated Fatty Liver Disease. Journal of Agricultural and Food Chemistry, 72(18), 10391-10405. DOI: 10.1021/acs.jafc.4c00590
[34]. Ye, J., Zheng, J., Tian, X., Xu, B., Yuan, F., Wang, B., and Huang, F., 2022, Fucoxanthin attenuates free fatty acid-induced nonalcoholic fatty liver disease by regulating lipid metabolism/oxidative stress/inflammation via the AMPK/Nrf2/TLR4 signaling pathway. Marine Drugs, 20(4), 225. https://doi.org/10.3390/md20040225
[35]. Koshak, M. F., El-Readi, M. Z., Elzubier, M. E., Refaat, B., Almaimani, R. A., Idris, S., and Eid, S. Y., 2023, Antioxidative and Anti-Inflammatory Protective Effects of Fucoxanthin against Paracetamol-Induced Hepatotoxicity in Rats. Marine Drugs, 21(11), 592. https://doi.org/10.3390/md21110592
[36]. Ben Ammar, R., Zahra, H. A., Abu Zahra, A. M., Alfwuaires, M., Abdulaziz Alamer, S., Metwally, A. M., and Al-Ramadan, S. Y., 2023, Protective effect of fucoxanthin on zearalenone-induced hepatic damage through Nrf2 mediated by PI3K/AKT signaling. Marine Drugs, 21(7), 391. https://doi.org/10.3390/md21070391
[37]. Winarto, J., Song, D. G., and Pan, C. H., 2023, The role of fucoxanthin in non-alcoholic fatty liver disease. International Journal of Molecular Sciences, 24(9), 8203. https://doi.org/10.3390/ijms24098203
[38]. Winarto, J., Song, D. G., and Pan, C. H., 2023, The role of fucoxanthin in non-alcoholic fatty liver disease. International Journal of Molecular Sciences, 24(9), 8203. https://doi.org/10.3390/ijms24098203
[39]. Bae, M., Kim, M. B., and Lee, J. Y., 2022, Fucoxanthin attenuates the reprogramming of energy metabolism during the activation of hepatic stellate cells. Nutrients, 14(9), 1902. https://doi.org/10.3390/nu14091902
[40]. Delgado-Ramallo, J. F., Ceballos-Cuevas, L., Álvarez-Gil, M., Suárez-Montes, D., Casado-Bañares, V., Goñi-de-Cerio, F., and Rodríguez, E., 2023, Phaeodactylum tricornutum as Fucoxanthin Biofactory Model and Hepatoprotective Effect of Encapsulated Spirulina and Fucoxanthin. Applied Sciences, 13(13), 7794. https://doi.org/10.3390/app13137794
[41]. Hong, D. D., Thom, L. T., Ha, N. C., Thu, N. T. H., Hien, H. T. M., Tam, L. T., ... and Ambati, R. R., 2023, Isolation of Fucoxanthin from Sargassum oligocystum Montagne, 1845 Seaweed in Vietnam and Its Neuroprotective Activity. Biomedicines, 11(8), 2310. https://doi.org/10.3390/biomedicines11082310
[42]. Ha, N. C., Thu, N. T. H., Hien, H. T. M., Tam, L. T., Duc, T. M., Van Tru, N., and Hong, D. D., 2023, Elucidation of Antioxidant and Neuroprotective Potential of Fucoxanthin Isolated from Brown Seaweed Sargassum oligocystum. 24 March 2023, PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-2721377/v1]
[43]. Lian, W., Hu, X., Zhang, J., Wu, Y., Zhao, N., Ma, H., and Lu, Q., 2023, Fucoxanthin protects retinal ganglion cells and promotes parkin-mediated mitophagy against glutamate excitotoxicity. Neuroreport, 34(7), 385-394. DOI: 10.1097/WNR.0000000000001902
[44]. Lee, N., Youn, K., Yoon, J. H., Lee, B., Kim, D. H., and Jun, M., 2023, The role of fucoxanthin as a potent Nrf2 activator via Akt/GSK-3β/Fyn axis against amyloid-β peptide-induced oxidative damage. Antioxidants, 12(3), 629. https://doi.org/10.3390/antiox12030629
[45]. Ferdous, K. A., Burnett, G., Scott, M., Amjad, E., Bannerman, S., and Park, H. A., 2022, Neuroprotective Function of Fucoxanthin in Oxidative Stress-Mediated Mitochondrial Dysfunction. Current Developments in Nutrition, 6, 787. https://doi.org/10.1093/cdn/nzac064.006
[46]. Chen, Y., Lu, H., Ding, Y., Liu, S., Ding, Y., Lu, B., and Zhou, X., 2023, Dietary protective potential of fucoxanthin as an active food component on neurological disorders. Journal of Agricultural and Food Chemistry, 71(8), 3599-3619. https://doi.org/10.1093/cdn/nzac064.006
[47]. Mao, H., Wang, L., Xiong, Y., Jiang, G., and Liu, X., 2022, Fucoxanthin attenuates oxidative damage by activating the Sirt1/Nrf2/HO‐1 signaling pathway to protect the kidney from ischemia‐reperfusion injury. Oxidative Medicine and Cellular Longevity, (1), 7444430. https://doi.org/10.1155/2022/7444430
[48]. Hudlikar, R. R., Sargsyan, D., Li, W., Wu, R., Zheng, M., and Kong, A. N., 2021, Epigenomic, transcriptomic, and protective effect of carotenoid fucoxanthin in high glucose-induced oxidative stress in Mes13 kidney mesangial cells. Chemical Research in Toxicology, 34(3), 713-722. https://doi.org/10.1021/acs.chemrestox.0c00235
[49]. El Bakary, N. M., Thabet, N. M., El Fatih, N. M., Abdel-Rafei, M. K., El Tawill, G., and Azab, K. S.,2021, Fucoxanthin alters the apelin-13/APJ pathway in certain organs of γ-irradiated mice. Journal of Radiation Research, 62(4), 600-617. https://doi.org/10.1093/jrr/rraa141
[50]. Bharathiraja, K., Babu, L. H., Vijayaprakash, S., Tamilselvan, P., and Balasubramanian, M. P., 2013, Fucoxanthin, a marine carotenoid protects cadmium-induced oxidative renal dysfunction in rats. Biomedicine & Preventive Nutrition, 3(3), 201-207. https://doi.org/10.1016/j.bionut.2013.04.005
[51]. Ou, H. C., Chou, W. C., Chu, P. M., Hsieh, P. L., Hung, C. H., and Tsai, K. L., 2019, Fucoxanthin protects against oxLDL‐induced endothelial damage via activating the AMPK‐Akt‐CREB‐PGC1α pathway. Molecular nutrition & food research, 63(10), 1801353. https://doi.org/10.1002/mnfr.201801353
[52]. Chiang, Y. F., Chen, H. Y., Chang, Y. J., Shih, Y. H., Shieh, T. M., Wang, K. L.,and Hsia, S. M., 2020, Protective effects of fucoxanthin on high glucose-and 4-hydroxynonenal (4-HNE)-induced injury in human retinal pigment epithelial cells. Antioxidants, 9(12), 1176. https://doi.org/10.3390/antiox9121176
[53]. Wang, W., Fu, C., Lin, M., Lu, Y., Lian, S., Xie, X., and Huang, M., 2022, Fucoxanthin prevents breast cancer metastasis by interrupting circulating tumor cells adhesion and transendothelial migration. Frontiers in Pharmacology, 13, 960375. https://doi.org/10.3389/fphar.2022.960375
[54]. Lee, C. Y., Chen, S. P., Huang‐Liu, R., Gau, S. Y., Li, Y. C., Chen, C. J., and Kuan, Y. H., 2022, Fucoxanthin decreases lipopolysaccharide‐induced acute lung injury through the inhibition of RhoA activation and the NF‐κB pathway. Environmental Toxicology, 37(9), 2214-2222. https://doi.org/10.1002/tox.23587
[55]. Yang, M., Xuan, Z., Wang, Q., Yan, S., Zhou, D., Naman, C. B., and Cui, W., 2022, Fucoxanthin has potential for therapeutic efficacy in neurodegenerative disorders by acting on multiple targets. Nutritional Neuroscience, 25(10), 2167-2180. https://doi.org/10.1002/tox.23587
[56]. Yoshiko, S., and Hoyoku, N., 2007, Fucoxanthin, a natural carotenoid, induces G1 arrest and GADD45 gene expression in human cancer cells. In vivo, 21(2), 305-310.
[57]. Mohamed Abdoul-Latif, F., Ainane, A., Houmed Aboubaker, I., Merito Ali, A., Mohamed, H., Jutur, P. P., and Ainane, T., 2024, Unlocking the Green Gold: Exploring the Cancer Treatment and the Other Therapeutic Potential of Fucoxanthin Derivatives from Microalgae. Pharmaceuticals, 17(7), 960. https://doi.org/10.3390/ph17070960
[58]. Li, D., Liu, Y., Liu, Y., and Wang, S., 2022, Encapsulation of fucoxanthin in fatty acid-bovine serum albumin micelles to improve the stability, bioavailability, and bioefficacy. Colloids and Surfaces B: Biointerfaces, 220, 112951.https://doi.org/10.1016/j.colsurfb.2022.112951
[59]. Liu, Y., Qiao, Z., Liu, W., Hou, Z., Zhang, D., Huang, L., and Zhang, Y.,2019, Oleic acid as a protein ligand improving intestinal absorption and ocular benefit of fucoxanthin in water through protein-based encapsulation. Food & Function, 10(7), 4381-4395. https://doi.org/10.1039/C9FO00814D
Viewed PDF 313 28 -
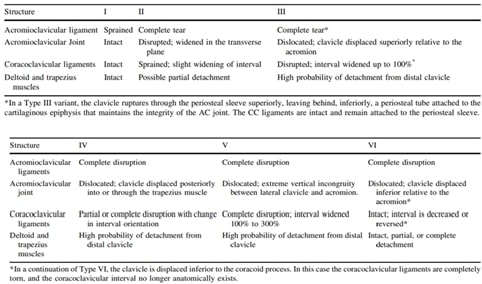 Post-surgical Functional Assessment Following Three Different Types of Surgical Repair in Type III to VI Acromioclavicular Joint DisruptionAuthor: Akshay J KumarDOI: 10.21522/TIJPH.2013.SE.24.05.Art039
Post-surgical Functional Assessment Following Three Different Types of Surgical Repair in Type III to VI Acromioclavicular Joint DisruptionAuthor: Akshay J KumarDOI: 10.21522/TIJPH.2013.SE.24.05.Art039Post-surgical Functional Assessment Following Three Different Types of Surgical Repair in Type III to VI Acromioclavicular Joint Disruption
Abstract:
This study assesses the functional outcomes and associated risks of various surgical procedures for types III to VI acromioclavicular (AC) joint injuries, including Endo button flipping, hamstring grafting, and the suture anchor & eight plate method. Conducted at Saveetha Medical College Hospitals from June 2021 to June 2022, the study involved 21 patients aged 25 to 60 with AC joint injuries, excluding types I and II injuries and those with medical ineligibility. Surgical correction included reconstructing the coracoclavicular ligaments using various techniques, followed by at least one year of follow-up. Functional outcomes were measured using the Constant Murley Score, and radiographic evaluations were performed at set intervals. The mean Constant scores were 92.54, 90.85, and 91.42 for the suture anchor, Endo button flip, and hamstring graft methods, respectively. Although the suture anchor technique had a slightly higher score, the difference was not statistically significant. All methods maintained coracoclavicular distance and provided a good range of motion. Patients treated with suture anchors showed notably fewer complications during the one-year follow-up. In conclusion, all surgical methods resulted in successful outcomes for AC joint injuries of types III to VI, with the suture anchor technique showing a trend toward better functional scores, though without significant differences. Further long-term monitoring is recommended to determine the optimal treatment approach.
Post-surgical Functional Assessment Following Three Different Types of Surgical Repair in Type III to VI Acromioclavicular Joint Disruption
References:
[1]. Pallis, M., Cameron, K. L., Svoboda, S. J., & Owens, B. D.,2012, Epidemiology of Acromioclavicular Joint Injury in Young Athletes. The American Journal of Sports Medicine, 40(9), 2072–2077, https://doi.org/10.1177/0363546512450162
[2]. Bishop, J. Y., & Kaeding, C., 2006, Treatment of the Acute Traumatic Acromioclavicular Separation. Sports Medicine and Arthroscopy Review, 14(4), 237–245. https://doi.org/10.1097/01.jsa.0000212330.32969.6e
[3]. Verstift, D. E., Kilsdonk, I. D., Van Wier, M. F., Haverlag, R., & Van Den Bekerom, M. P., 2021b, Long-term Outcome After Nonoperative Treatment for Rockwood I and II Acromioclavicular Joint Injuries. The American Journal of Sports Medicine, 49(3), 757–763. https://doi.org/10.1177/0363546520981993
[4]. Verstift, D. E., Kilsdonk, I. D., Van Wier, M. F., Haverlag, R., & Van Den Bekerom, M. P., 2021c, Long-term Outcome After Nonoperative Treatment for Rockwood I and II Acromioclavicular Joint Injuries. The American Journal of Sports Medicine, 49(3), 757–763, https://doi.org/10.1177/0363546520981993
[5]. Verstift, D. E., Welsink, C. L., Spaans, A. J., & Van Den Bekerom, M. P. J., 2019, Return to sport after surgical treatment for high-grade (Rockwood III–VI) acromioclavicular dislocation. Knee Surgery Sports Traumatology Arthroscopy, 27(12), 3803–3812, https://doi.org/10.1007/s00167-019-05528-w
[6]. Baren J. P., Rowbotham.E, Robinson, P., 2022, Acromioclavicular Joint Injury and Repair. Semin Musculoskelet Radiol, 26:597–610.
[7]. de Groot, C., Verstift, D. E., Heisen, J., van Deurzen, D. F. P., van den Bekerom, 2023, MPJ. Management of Acromioclavicular Injuries – Current Concepts. Orthop Res Rev,15:1-12
https://doi.org/10.2147/ORR.S340531Bannister GC, Wallace WA, Stableforth PG, et al. A classification of acute acromioclavicular dislocation: A clinical, radiological, and anatomical study. Injury. 1992, 23, 194–196[8]. Gorbaty, J. D., Hsu, J. E., Gee, A. O., 2017, Classifications in Brief: Rockwood Classification of Acromioclavicular Joint Separations. Clin Orthop Relat Res. Jan, 475(1), 283-287. doi: 10.1007/s11999-016-5079-6. Epub 2016 Sep 16. PMID: 27637619; PMCID: PMC5174051. Rockwood CA, Williams, G. R., Young, D. C., Disorders of the acromioclavicular joint. In: Rockwood CA, Matsen FA, editors. The Shoulder. Philadelphia: Saunders; 1998:483–553.
[9]. Boffano, M., Mortera, S., Wafa, H., Piana, R., 2017, The surgical treatment of acromioclavicular joint injuries. EFORT Open Rev, Oct 19, 2(10), 432-437. doi: 10.1302/2058-5241.2.160085. PMID: 29209519; PMCID: PMC5702953.
[10]. Sirin, E., Aydin, N., Mert Topkar, O., 2018, Acromioclavicular joint injuries: Diagnosis, classification and ligamentoplasty procedures. EFORT Open Rev. Jul 17, 3(7), 426-433, doi: 10.1302/2058-5241.3.170027. PMID: 30233818; PMCID: PMC6129955.Fraser-Moodie JA, Shortt NL, Robinson CM. Injuries to the acromioclavicular joint. J Bone Joint Surg Br 2008, 90(06), 697–707.
[11]. Warth, R. J., Martetschläger, F., Gaskill, T. R., Millett, P. J., 2013, Acromioclavicular joint separations. Curr Rev Musculoskelet Med., 6(1), 71–78. doi:10.1007/s12178-012-9144-9.
[12]. Lee, S. J., Nicholas, S. J., Akizuki, K. H., McHugh, M. P., Kremenic, I. J., Ben-Avi, S., 2003, Reconstruction of the coracoclavicular ligaments with tendon grafts: a comparative biomechanical study. Am J Sports Med. Sep-Oct; 31(5), 648-55, doi: 10.1177/03635465030310050301. PMID: 12975181.
[13]. Zhang, L., Zhou, X., Qi, J., Zeng, Y., Zhang, S., Liu, G., Ping, R., Li, Y., Fu, S., 2018, Modified closed-loop double-endobutton technique for repair of rockwood type III acromioclavicular dislocation. Exp Ther Med. Jan, 15(1), 940-948. doi: 10.3892/etm.2017.5487. Epub 2017 Nov 10. PMID: 29399102; PMCID: PMC5772745.
[14]. Özden, Raif., 2014, Endobutton technique for the treatment of acute acromioclavicular joint dislocations. Dicle Medical Journal/Dicle Tıp Dergisi. 41. 268-271. 10.5798/diclemedj.0921.2014.02.0414.
[15]. Virtanen, K. J., Savolainen, V., Tulikoura, I., Remes, V., Haapamäki, V., Pajarinen, J., Björkenheim, J. M., Paavola, M., 2014, Surgical treatment of chronic acromioclavicular joint dislocation with autogenous tendon grafts. Springerplus. Aug 10, 3, 420. Doi: 10.1186/2193-1801-3-420. PMID: 25152850; PMCID: PMC4141074.
[16]. Gorbaty, J. D., Hsu, J. E., Gee, A. O., 2017, Classifications in Brief: Rockwood Classification of Acromioclavicular Joint Separations. Clin Orthop Relat Res. 475(1), 283-287. doi: 10.1007/s11999-016-5079-6. Epub 2016 Sep 16. PMID: 27637619; PMCID: PMC5174051.
[17]. Fukuda, K., Craig, E. V., An, K. N., et al., 1986, Biomechanical study of the ligamentous system of the acromioclavicular joint. J Bone Joint Surg., 68(3), 434–440.
[18]. Lee, K. W., Debski, R. E., Chen, C. H., et al., 1997, Functional evaluation of the ligaments at the acromioclavicularjoint during anteroposterior and superoinferior translation. Am J Sports Med., 25, 858–862.
[19]. Rockwood, C. A., Williams, G. R., Young, D. C., 2004, Disorders of the acromio-clavicular joint. In: Rockwood CA, Matsen FA, eds. The Shoulder. 3rd ed. Philadelphia, PA: WB, Saunders Co, 521–586.
[20]. Tossy, J., Newton, C. M., Sigmond, H. M., 1963, 11 acromioclavicular separations: Useful and practical classification for treatment. Clin Orthop Relat Res., 28, 111–119.
[21]. Steven Struhl, Theodore S. Wolfson, 2016, Closed-Loop Double Endobutton Technique for Repair of Unstable Distal Clavicle Fractures, The Orthopaedic Journal of Sports Medicine, 4(7), 2325967116657810, DOI: 10.1177/2325967116657810
[22]. Manohara, R., Reid, J. T., 2019, Percutaneous endobutton fixation of acute acromioclavicular joint injuries and lateral clavicle fractures. J Clin Orthop Trauma. May-Jun;10(3):492-496. doi: 10.1016/j.jcot.2018.10.013. Epub 2018 Oct 21. PMID: 31061575; PMCID: PMC6494760.
[23]. Struhl, S., Wolfson, T. S., 2015, Continuous Loop Double Endobutton Reconstruction for Acromioclavicular Joint Dislocation. The American Journal of Sports Medicine, 43(10), 2437-2444. doi:10.1177/0363546515596409
[24]. Liu, T., Bao, F. L., Jiang, T., Ji, G. W., Li, J. M., Jerosch, J., 2020, Acromioclavicular Joint Separation: Repair Through Suture Anchors for Coracoclavicular Ligament and Nonabsorbable Suture Fixation for Acromioclavicular Joint. Orthop Surg. Oct;12(5):1362-1371. doi: 10.1111/os.12771. Epub 2020 Sep 6. PMID: 32893498; PMCID: PMC7670157.
[25]. Raju H Kulkarni, Sharan Chennur, 2021, Functional outcome of acromioclavicular joint disruption treated using anchor sutures. Int. J. Orthop. Sci., 7(4), 696-699. DOI: 10.22271/ortho.2021.v7.i4j.2954
[26]. Virtanen, K. J., Savolainen, V., Tulikoura, I., Remes, V., Haapamäki, V., Pajarinen, J., Björkenheim, J. M., Paavola, M., 2014,Surgical treatment of chronic acromioclavicular joint dislocation with autogenous tendon grafts. Springerplus. Aug 10, 3, 420. doi: 10.1186/2193-1801-3-420. PMID: 25152850; PMCID: PMC4141074.
[27]. Dhilip, Ashita, Parameswari, R. P., 2024, Deciphering the Involvement of Chronic Inflammation in Osteoarthritis: Evaluation of Complement 3 and Cathepsin D in Osteoarthritic Patients—A Retrospective Case Study. Journal of Pharmacy and Bioallied Sciences 16(Suppl 2), p S1321-S1325, April 2024, DOI: 10.4103/jpbs.jpbs_539_23
[28]. Sekar, N., & Subash, Y., 2024, Assessing Initial Functional Outcomes Following Arthroscopic Release for Stiff Shoulder in Periarthritis Shoulder. South Eastern European Journal of Public Health, 600–605. https://doi.org/10.70135/seejph.vi.1220
[29]. Sugumar, N., Sathiyaseelan, N., Purushothaman, J. R. et al., 2024, Assessing functional and radiological outcomes: open reduction and internal fixation vs. minimally invasive plate osteosynthesis for humerus shaft fractures - a prospective comparative study. International Orthopaedics (SICOT), https://doi.org/10.1007/s00264-024-06307-0
Viewed PDF 207 7 -
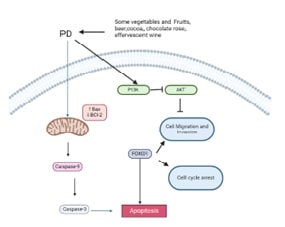 Polydatin: A Promising Natural Agent with Anti-Hepatocellular Carcinoma PropertiesAuthor: Nallusamy DuraisamyDOI: 10.21522/TIJPH.2013.SE.24.05.Art040
Polydatin: A Promising Natural Agent with Anti-Hepatocellular Carcinoma PropertiesAuthor: Nallusamy DuraisamyDOI: 10.21522/TIJPH.2013.SE.24.05.Art040Polydatin: A Promising Natural Agent with Anti-Hepatocellular Carcinoma Properties
Abstract:
The review extensively examines the multifaceted anti-cancer properties of polydatin (PD), a stilbenoid compound sourced from fruits and vegetables. PD is known for its antioxidant capabilities, anti-inflammatory effects, and anti-cancer properties. The analysis delves into PD's impact on cancer characteristics such as cellular proliferation, metastasis, and apoptosis. It highlights PD's potential as a targeted therapeutic agent and its synergistic interactions with existing anti-cancer medications, aiming to enhance understanding and innovative strategies in cancer therapy. Ultimately, this review aims to provide a comprehensive analysis of PD's diverse anti-cancer attributes and intrinsic value in advancing novel paradigms in cancer treatment and prevention, instilling hope for the future of cancer therapy.
Polydatin: A Promising Natural Agent with Anti-Hepatocellular Carcinoma Properties
References:
[1]. Jun, J.I., Lau, L.F., 2018, Resolution of organ fibrosis. Journal of Clinical Investigation. 128(1):97-107. Doi:10.1172/JCI93563
[2]. Cheong, K.L., Yu, B., & Teng, B., 2023, Post-COVID-19 syndrome management: Utilizing the potential of dietary polysaccharides. Biomedicine & Pharmacotherapy. 166(115320):115320. Doi: 10.1016/j.biopha.2023.115320
[3]. Cheong, K.L., Chen, S., Teng, B., Veeraperumal, S., Zhong, S., & Tan. K., 2023, Oligosaccharides as potential regulators of gut Microbiota and intestinal health in post-COVID-19 management. Journal Pharmaceuticals (Basel). 16(6). Doi:10.3390/ph16060860.
[4]. Wang, M., Veeraperumal, S., Zhong, S., & Cheong, K.L., 2023, Fucoidan-derived functional oligosaccharides: Recent developments, preparation, and potential applications. Foods. 12(4). Doi:10.3390/foods12040878
[5]. Tang, C., Ding, R., Sun, J., Liu, J., & Kan, J., 2019, The impacts of natural polysaccharides on intestinal microbiota and immune responses-a review. Journal Food & Function is Food & Function. 10:2290-2312.
[6]. Zhang, A., Wang, J., Hu, Y., Qiu, Y., & Dong, C., 2024, Polysaccharides play an anti-fibrotic role by regulating intestinal flora: A review of research progress. International Journal of Biological Macromolecules. 271(Pt 2):131982. Doi: 10.1016/j.ijbiomac.2024.131982
[7]. Wang, M., Lu, S., Zhao, H., Liu, Z., Sheng, K., & Fang, J., 2022, Natural polysaccharides as potential anti-fibrotic agents: A review of their progress. Journal Life Sciences. 308(120953):120953. Doi: 10.1016/j.lfs.2022.120953
[8]. Zhang, L-Y LZ., 2020, Advances in the research of anti-organ fibrosis drugs. Acta Pharmaceutica Sinica B. 2510-2528.
[9]. Zhao, X., Chen, J., Sun, H., Zhang, Y., Zou, D., 2022, New insights into fibrosis from the ECM degradation perspective: the macrophage-MMP-ECM interaction. Journal Cell & Bioscience. 12(1):117. Doi:10.1186/s13578-022-00856-w
[10]. Rockey, D.C., Bell, P.D., Hill, J.A., 2015, Fibrosis-a common pathway to organ injury and failure. The New England Journal of Medicine. 372:1138-1149
[11]. Zhao, M., Wang, L., Wang, M., Zhou, S., Lu, Y., 2022, Targeting fibrosis: Mechanisms and clinical trials. Signal transduction and Targeted Therapy. 7
[12]. Kozawa, S., Tejima, K., Takagi, S., 2023, Latent inter-organ mechanism of idiopathic pulmonary fibrosis unveiled by a generative computational approach. Scientific Reports. 13(1):21981. Doi:10.1038/s41598-023-49281-0
[13]. Álvarez, J., Real, J., Guarner, F., Gueimonde, M., Rodríguez, J.M., 2021, Microbiota intestinal y salud. Gastroenterología y Hepatología. 44:519-535
[14]. Wu, Y., Li, Y., Luo, Y., 2022, Gut microbiome and metabolites: The potential key roles in pulmonary fibrosis. Frontiers in Microbiology. 13:943791. Doi:10.3389/fmicb.2022.943791
[15]. Drakopanagiotakis, F., Stavropoulou, E., Tsigalou, C., Nena, E., Steiropoulos, P., 2022 The role of the microbiome in connective-tissue-associated interstitial lung disease and pulmonary vasculitis. Biomedicines. 10(12):3195. Doi:10.3390/biomedicines10123195
[16]. Chioma, O.S., Mallott, E.K., Chapman, A., 2022, Gut microbiota modulates lung fibrosis severity following acute lung injury in mice. Communications Biology. 5(1):1401. Doi:10.1038/s42003-022-04357-x
[17]. Aydın, M.M., Akçalı, K.C., 2018, Liver fibrosis. Turkish Journal of Gastroenterology. 29(1):14-21. Doi:10.5152/tjg.2018.17330
[18]. Trautwein, C., Friedman, S.L., Schuppan, D., Pinzani, M., 2015, Hepatic fibrosis: Concept to treatment. Hepatology. 62(1 Suppl):S15-24. Doi: 10.1016/j.jhep.2015.02.039
[19]. Friedman, S.L., 2024, Hepatic fibrosis and cancer: The silent threats of metabolic syndrome. Diabetes Metab J. 48(2):161-169. Doi:10.4093/dmj.2023.0240
[20]. Lee, C.M., Yoon, E.L., Kim, M., 2024, Prevalence, distribution, and hepatic fibrosis burden of the different subtypes of steatotic liver disease in primary care settings. Hepatology. 79(6):1393-1400. Doi:10.1097/HEP.0000000000000664
[21]. Gao, L.L., Ma, J.M., Fan, Y.N., 2021, Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. International Journal of Biological Macromolecules. 183:1379-1392. Doi: 10.1016/j.ijbiomac.2021.05.066
[22]. Li S. 2022. Investigation of the Anti-HF Mechanism of Taraxacum mongolicum Polysaccharide With Astragalus Polysaccharide Via Gut–Liver Axis.
[23]. Shu, Y., Huang, Y., Dong, W., 2023, The polysaccharides from Auricularia auricula alleviate non-alcoholic fatty liver disease via modulating gut microbiota and bile acids metabolism. International Journal of Biological Macromolecules. 246(125662):125662. Doi: 10.1016/j.ijbiomac.2023.125662
[24]. Han, C., Li, Z., Liu, R., 2023, Lonicerae flos polysaccharides improve nonalcoholic fatty liver disease by activating the adenosine 5’-monophosphate-activated protein kinase pathway and reshaping gut microbiota. Journal of the Science of Food and Agriculture. 103(15):7721-7738. Doi:10.1002/jsfa.12854
[25]. Fang, S., Wang, T., Li, Y., 2022, Gardenia jasminoides Ellis polysaccharide ameliorates cholestatic liver injury by alleviating gut microbiota dysbiosis and inhibiting the TLR4/NF-κB signaling pathway. International Journal of Biological Macromolecules 205:23-36. Doi: 10.1016/j.ijbiomac.2022.02.056
[26]. Humphreys, B.D., 2018, Mechanisms of renal fibrosis. Annual Review of Physiology. 80(1):309-326. Doi:10.1146/annurev-physiol-022516-034227
[27]. Liu, Y., 2011, Cellular and molecular mechanisms of renal fibrosis. Nature Reviews Nephrology. 7(12):684-696. Doi:10.1038/nrneph.2011.149
[28]. Nogueira, A., Pires, M.J., Oliveira, P.A., 2017, Pathophysiological mechanisms of renal fibrosis: A review of animal models and therapeutic strategies. In Vivo. 31(1):1-22. Doi:10.21873/invivo.11019
[29]. Yang, J., Dong, H., Wang, Y., 2020, Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. International Journal of Biological Macromolecules. 163:442-456. Doi: 10.1016/j.ijbiomac.2020.06.153
[30]. Zhang, M., Yang, L., Zhu, M., 2022, Moutan Cortex polysaccharide ameliorates diabetic kidney disease via modulating gut microbiota dynamically in rats. International Journal of Biological Macromolecules. 206:849-860. Doi: 10.1016/j.ijbiomac.2022.03.077
[31]. Feng, Y., Weng, H., Ling, L., 2019, Modulating the gut microbiota and inflammation is involved in the effect of Bupleurum polysaccharides against diabetic nephropathy in mice. International Journal of Biological Macromolecules. 132:1001-1011. Doi: 10.1016/j.ijbiomac.2019.03.242
[32]. Liu, J.X., Yuan, H.Y., Li, Y.N., Wei, Z., Liu, Y., Liang, J., 2022, Ephedra sinica polysaccharide alleviates airway inflammations of mouse asthma-like induced by PM2. 5 and ovalbumin via the regulation of gut microbiota and short chain fatty acid. Journal of Pharmacy and Pharmacology. 74:1784-1796.
[33]. Shi, C., Zhou, L., Li, H., 2022, Intestinal microbiota metabolizing Houttuynia cordata polysaccharides in H1N1 induced pneumonia mice contributed to Th17/Treg rebalance in gut-lung axis International Journal of Biological Macromolecules. 221:288-302. Doi: 10.1016/j.ijbiomac.2022.09.015
[34]. Long, H.Z., Cheng, Y., Zhou, Z.W., Luo, H.Y., Wen, D.D., Gao, L.C., 2021, PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer's Disease and Parkinson's Disease. Frontiers in Pharmacology. 12:648636. Doi:10.3389/fphar.2021.648636
Viewed PDF 203 8 -
 Insight on the Health Benefits of Functional Food: Dark ChocolateAuthor: Nallusamy DuraisamyDOI: 10.21522/TIJPH.2013.SE.24.05.Art041
Insight on the Health Benefits of Functional Food: Dark ChocolateAuthor: Nallusamy DuraisamyDOI: 10.21522/TIJPH.2013.SE.24.05.Art041Insight on the Health Benefits of Functional Food: Dark Chocolate
Abstract:
Dark chocolate is known for its rich content of bioactive compounds such as polyphenols and flavonoids, which possess potent antioxidant properties that counteract oxidative stress and lower the risk of chronic diseases like cardiovascular diseases and certain cancers. Studies have shown that dark chocolate consumption can improve cardiovascular health by enhancing endothelial function and reducing blood pressure. Mendelian randomization studies have further indicated a potential decrease in the risk of essential hypertension and venous thromboembolism. Additionally, dark chocolate may favour lipid metabolism, weight management, and inflammation in individuals with chronic kidney disease. There is also emerging evidence suggesting that dark chocolate could positively influence cognitive function and mood and offer protection against neurodegenerative diseases like Alzheimer's and Parkinson's. Researchers are exploring innovative fortification methods to enhance the nutritional profile of dark chocolate and maximize its health benefits. In this review, we have summarized the investigation of the association between dark chocolate consumption and its health-promoting effects.
Insight on the Health Benefits of Functional Food: Dark Chocolate
References:
[1]. Dehghani, P., Taheri, F., Asgary, S., 2024, Decoding the delights: Unraveling the health benefits of dark chocolate in comparison to white chocolate. Functional Food Science. 4:119-133.
[2]. Yang, J., Zhou, J., Yang, J., 2024, Dark chocolate intake and cardiovascular diseases: A Mendelian randomization study. Scientific Reports. 14(1):968. Doi:10.1038/s41598-023-50351-6
[3]. Ferina, F., Hadianti, D.N., Fatimah, Y.U., 2023, Dark chocolate as a non-pharmacological alternative to reduce dysmenorrhea in adolescents. Healthcare in Low-resource Settings. 11.
[4]. Cartas, J., Alvarenga, N., Partidário, A., 2024, Influence of geographical origin in the physical and bioactive parameters of single origin dark chocolate. European Food Research and Technology 250(10):2569-2580. Doi:10.1007/s00217-024-04558-0
[5]. Kerimi, A., Williamson, G., 2015, The cardiovascular benefits of dark chocolate. Vascular Pharmacology. 71:11-15. Doi:10.1016/j.vph.2015.05.011
[6]. Zugravu, C., Otelea, M.R., 2019, Dark chocolate: To eat or not to eat? A review. Journal of AOAC International. 102(5):1388-1396. Doi:10.1093/jaoac/102.5.1388
[7]. Pierce, E.A., 2024, The Health Effects of Chocolate: A Literature Review.
[8]. Singh, P.K., Khedkar, R.D., Chandra, S., 2024, Chocolate: An overview of functional potential and recent trends in fortification. Brazilian Journal of Food Technology. 27. Doi:10.1590/1981-6723.11823
[9]. Samanta, S., Sarkar, T., Chakraborty, R., 2022, Dark chocolate: An overview of its biological activity, processing, and fortification approaches. Current Research in Food Science. 5:1916-1943. Doi: 10.1016/j.crfs.10.017
[10]. Jaćimović, S., Popović-Djordjević, J., Sarić, B., Krstić, A., Mickovski-Stefanović, V., Pantelić, N.Đ., 2022 Antioxidant activity and multi-elemental analysis of dark chocolate. Foods. 11(10):1445. Doi:10.3390/foods11101445
[11]. Martins LM, de Santana LRR, Maciel LF, Soares SE, Ferreira ACR, et al. 2023. Phenolic compounds, methylxanthines, and preference drivers of dark chocolate made with hybrid cocoa beans.
[12]. Vinci, G., Maddaloni, L., Prencipe, S.A., Orlandini, E., Sambucci, M., 2023 Simple and reliable eco‐extraction of bioactive compounds from dark chocolate by Deep Eutectic Solvents. A sustainable study. International Journal of Food Science and Technology. Doi:10.1111/ijfs.16315
[13]. Mori Culqui, P.L., Quintana, C., 202, 1Antioxidantes y polifenoles totales de chocolate negro con incorporación de cacao (Theobroma cacao L.) crudo. Revista de Investigaciones Altoandinas. 23:266-273.
[14]. Sasaki, A., Mizuno, K., Morito, Y., 2024, The effects of dark chocolate on cognitive performance during cognitively demanding tasks: A randomized, single-blinded, crossover, dose-comparison study. Heliyon. 10(2): e24430. Doi: 10.1016/j.heliyon.2024.e24430
[15]. Cinquanta, L., Di Cesare, C., Manoni, R., Piano, A., Roberti, P., Salvatori, G., 2016, Mineral essential elements for nutrition in different chocolate products. International Journal of Food Sciences and Nutrition. 67(7):773-778. Doi:10.1080/09637486.2016.1199664
[16]. Singh, R., Deshpande, A.S., Pallavi, A., Kamboj, V., 2023, Sustainable Use of Waste Banana Peel (Musa× sapientum L.) Powder for Enhancement of Nutritional Properties of Dark Chocolate. Agro Environmental Sustainability is AgroEnviron. 1(1):22-28.
[17]. Monteiro, S., Dias, J., Lourenço, V., 2023, Development of a functional dark chocolate with baobab pulp. Foods. 12(8). Doi:10.3390/foods12081711
[18]. Rashid, A., Misson, S., Yaakob, M., Latiff, H., Sarmidi, N.A., 2017, Addition of virgin coconut oil: Influence on the nutritional value and consumer acceptance of dark chocolate. Journal Science China Technological Sciences. 4(3-3):426-431.
[19]. Berroukche, A., Terras, M., Dellaoui, H., 2018, Theobromine dark chocolate extract and vitamin A antagonist effects on biochemical parameters in rats fed with high-fat diet. Biochemistry and Molecular Biology Journals. 04(03). Doi:10.21767/2471-8084.100071
[20]. Tan, T.Y.C., Lim, X.Y., Yeo, J.H.H., Lee, S.W.H., Lai, N.M., 2021, The health effects of chocolate and cocoa: A systematic review. Nutrients. 13(9):2909. Doi:10.3390/nu13092909
[21]. De, P., Silva, T., Silva, A.A., Toffolo, M., De Aguiar, A.S., 2022, The action of phytochemicals present in cocoa in the prevention of vascular dysfunction and atherosclerosis. Journal of Clinical and Translational Research. 8(6).
[22]. Ribeiro, M., Fanton, S., Paiva, B.R., 2023, Dark chocolate (70% cocoa) attenuates the inflammatory marker TNF-α in patients on hemodialysis. Clinical Nutrition ESPEN. 53:189-195. Doi: 10.1016/j.clnesp.12.009
[23]. Prenetha, R., Parameswari, R., Lakshmi, T., In vitro antioxidant and cytochrome p450 inhibitory activity of dark chocolate mediated zinc oxide nanoparticles.
[24]. García, L.C., Hernández, A.N.M., 2020, Beneficial effects of cocoa and dark chocolate polyphenols on health. Federation of American Societies for Experimental Biology. 34(S1):1-1. Doi:10.1096/fasebj.2020.34.s1.07187
[25]. Alvarez, L., Contreras, J., Giraldo, M., 2020, An individual patient data meta-analysis with Colombian studies on the effect of dark chocolate consumption on cardiovascular risk parameters. Journal of Nutrition and Metabolism. 2020:3419598. Doi:10.1155/2020/3419598
[26]. Rusli, A.A., Mohamad, N.J., Mahmood, A., Ibrahim, N.H., 2023, Antioxidant Capacity, Alpha Amylase Inhibition, and Calorie Value of Dark Chocolate Substituted with Honey Powder. Pertanika Journal of Tropical Agricultural Science. 46(4).
[27]. Shahbazi, S., Didar, Z., Vazifedoost, M., Naji-Tabasi, S., 2022, Enrichment of dark chocolate with free and microencapsulated white tea and jujube extracts: Impacts on antioxidant, physicochemical, and textural properties. Quality Assurance and Safety of Crops & Foods. 14(4):188-201.
[28]. Tafurt, G., Suarez, O., Lares, M. del. C., Álvarez, C., Liconte, N., 2020, Capacidad antioxidante de un chocolate oscuro de granos cacao orgánico sin fermentar Revista Digital de Postgrado. 10(1). Doi:10.37910/rdp.2021.10.1.e280
[29]. Magrone, T., Russo, M.A., Jirillo, E., 2017, Cocoa and dark chocolate polyphenols: From biology to clinical applications. Frontiers in Immunology. 8:677. Doi:10.3389/fimmu.2017.00677
[30]. Mafra, D., M Ribeiro, M., Fanton, S., 2022, MO570: Effects of dark chocolate on inflammation and oxidative stress in patients with chronic kidney disease on hemodialysis. Nephrology, Dialysis, Transplantation. 37.Doi:10.1093/ndt/gfac074.01531.
[31]. Ellinger, S., Stehle, P., 2016, Impact of cocoa consumption on inflammation processes-A critical review of randomized controlled trials. Nutrients. 8(6):321. Doi:10.3390/nu8060321
[32]. Zeng, H., Locatelli, M., Bardelli, C., 2011, Anti-inflammatory properties of clovamide and Theobroma cacao phenolic extracts in human monocytes: evaluation of respiratory burst, cytokine release, NF-κB activation, and PPARγ modulation. Journal of Agricultural and Food Chemistry. 59(10):5342-5350. Doi:10.1021/jf2005386
[33]. Ioannone, F., Sacchetti, G., Serafini, M., 2017, Effect of dark chocolate extracts on phorbol 12-myristate 13-acetate-induced oxidative burst in leukocytes isolated by normo-weight and overweight/obese subjects. Frontiers in Nutrition. 4:23. Doi:10.3389/fnut.2017.00023
[34]. Massaro, M., Scoditti, E., Carluccio, M.A., Kaltsatou, A., Cicchella, A., 2019, Effect of cocoa products and its polyphenolic constituents on exercise performance and exercise-induced muscle damage and inflammation: A review of clinical trials. Nutrients. 11(7):1471. Doi:10.3390/nu11071471
[35]. Patel, N., Jayswal, S., Maitreya, B.B., 2019, Dark Chocolate: Consumption for human health. Journal of Pharmacognosy and Phytochemistry. 8(3):2887-2890.
[36]. Nemoto, K., Kokubun, K., Ogata, Y., Koike, Y., Arai, T., Yamakawa, Y., 2022, Dark chocolate intake may reduce fatigue and mediate cognitive function and gray matter volume in healthy middle-aged adults. Behavioural Neurology. 2022:6021811. Doi:10.1155/2022/6021811
[37]. Lamport, D.J., Christodoulou, E., Achilleos, C., 2020, Beneficial effects of dark chocolate for episodic memory in healthy young adults: A parallel-groups acute intervention with a white chocolate control. Nutrients. 12(2):483. Doi:10.3390/nu12020483
[38]. Sumiyoshi, E., Matsuzaki, K., Sugimoto, N., 2019, Sub-chronic consumption of dark chocolate enhances cognitive function and releases nerve growth factors: A parallel-group randomized trial. Nutrients. 11(11):2800. Doi:10.3390/nu11112800
[39]. Tuenter, E., Foubert, K., Pieters, L., 2018, Mood components in cocoa and chocolate: The mood pyramid. Planta Medica. 84(12-13):839-844. Doi:10.1055/a-0588-5534
[40]. Kalantarzadeh, E., Radahmadi, M., Reisi, P., 2020, Effects of different dark chocolate diets on memory functions and brain corticosterone levels in rats under chronic stress. Journal of physiology and pharmacology. 24(3):185-196. Doi:10.32598/ppj.24.3.40
[41]. Pase, M.P., Scholey, A.B., Pipingas, A., 2013, Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo-controlled trial. Journal of Psychopharmacology. 27(5):451-458. Doi:10.1177/0269881112473791
[42]. Scholey, A., Owen, L., 2013, Effects of chocolate on cognitive function and mood: a systematic review. Nutrition Reviews. 71(10):665-681. Doi:10.1111/nure.12065
[43]. Shateri, Z., Kooshki, A., Hormoznejad, R., Hosseini, S.A., Mousavi, R., Foroumandi, E., 2023, Effects of chocolate on cognitive function in healthy adults: A systematic review and meta-analysis on clinical trials. Phytotherapy Research. 37(9):3688-3697. Doi:10.1002/ptr.7896
[44]. Sacramento, A.M., Silva, H.S. da., Melo, G.F. de., Tavares, G.S., Abreu, J.N.S., Chariglione, I.P.F.S., 2022, Benefits of combined interventions for cognitive enhancement in older adults. Journal of Gerontology and Geriatrics. 16. Doi:10.53886/gga.e0220018
[45]. Banaei, P., Tadibi, V., Amiri, E., Machado, D.G. da. S., 2023, Concomitant dual-site tDCS and dark chocolate improve cognitive and endurance performance following cognitive effort under hypoxia: a randomized controlled trial. Scientific Reports. 13(1):16473. Doi:10.1038/s41598-023-43568-y
[46]. Vyas, C.M., Manson, J.E., Sesso, H.D., 2024, Effect of cocoa extract supplementation on cognitive function: results from the clinic subcohort of the COSMOS trial. American Journal of Clinical Nutrition. 119(1):39-48. Doi: 10.1016/j.ajcnut.2023.10.031
[47]. Anggi, A.R., Herlina, E.C., Pengaruh, Pemberian., 2016, Dark Chocolate Terhadap Jumlah Spermatozoa Mencit Balb/C Jantan yang Dipapar Asap Rokok. Jurnal Kedokteran Diponegoro (Diponegoro Medical Journal). 5(4):475-484.
[48]. Eslami, O., 2017, Dark Chocolate and Metabolic Syndrome; Do Polyphenols All that Matter? Journal of Nutrition and Food Security. 2(2):139-140.
[49]. Naghdipour, B.A., Mozaffari, K.H., Poursoleiman, F., Zavar, R.J., Rahmanian, M., Dehghani, A., 2014, The Effect of Dark Chocolate Consumption on Lipid Profile in Patients with Metabolic Syndrome: A Randomized Clinical Trial.
[50]. Novianto, E., Maria, L., Mumpuni, R.Y., 2022, Pengaruh Dark Chocolate Terhadap Penurunan Tekanan Darah Sistolik Pada Penderita Hipertensi Usia 45-55 Tahun di RW. 04 Desa Banjarejo Kecamatan Pakis Kabupaten Malang: The Effect of Dark Chocolate on Controlling Systolic Blood Pressure in Hypertensive Patients. Medica Hospitalia: Journal of Clinical Medicine. 9(2):122-129.
[51]. Haritha, K., Kalyani, L., Rao, A.L., 2014, Health benefits of dark chocolate. Journal of Advanced Drug Delivery. 1(4):184-195.
[52]. Belz, G., Mohr-Kahaly, S., 1946, Cacoa and dark chocolate in cardiovascular prevention? Deutsche Medizinische Wochenschrift. Deutsche Medizinische Wochenschrift. 136:2657-2663.
[53]. Nurhayati, R., Zulfa, N., Herawati, E.R.N., Laila, U., 2022, Physicochemical, and microbiological characteristics of probiotic dark chocolate bar sweetened with palm sugar and coconut sugar. Food Research. 6(5):97-107. Doi:10.26656/fr.2017.6(5).591
[54]. Ali, Laila, A.S., Nahed, M.E., Magda, S.S., 2021, Producing and quality attributes of low calories dark chocolate using different intense sweeteners and wheat fiber isolate. American Journal of Food Technology. 9(1):1-7. Doi:10.12691/ajfst-9-1-1
[55]. Kim, S.Y., 2014, Department of Food Engineering, Dankook University, Zu G, Kim BK. Quality characteristics of single origin bean-to-bar dark chocolate prepared with sugar alcohols. Food Engineering Progress. 18(3):194-202. Doi:10.13050/foodengprog.2014.18.3.194
[56]. Saputro, A.D., Van, de. Walle. D., Aidoo, R.P., 2017, Quality attributes of dark chocolates formulated with palm sap-based sugar as nutritious and natural alternative sweetener. European Food Research and Technology. 243(2):177-191. Doi:10.1007/s00217-016-2734-9
[57]. Lorenzo, N.D., Ovd, S., Scds, L., 2022, Structure and nutrition of dark chocolate with pequi mesocarp (Caryocar villosum (Alb.) Pers.). Food Science and Technology. 42.
[58]. Nazira, S., Azada, Z., 2016, Development of a novel calorie controlled and sugar-free dark chocolate enriched with guar gum. Journal of Food Research. 4(02):39-46.
[59]. Khuda, S.E., Williams, K.M., 2015, Effect of Processing on Dark Chocolate Composition: A Focus on Allergens. Processing and Impact on Active Components in Food. Elsevier.
[60]. Bignardi, C., Mattarozzi, M., Penna, A., 2013, A rapid size-exclusion solid-phase extraction step for enhanced sensitivity in multi-allergen determination in dark chocolate and biscuits by liquid chromatography–tandem mass spectrometry. Food Analytical Methods. 6(4):1144-1152. Doi:10.1007/s12161-012-9521-4
[61]. Petyaev, I.M., Bashmakov, Y.K., 2017, Dark chocolate: Opportunity for an alliance between medical science and the food industry? Frontiers in Nutrition. 4:43. Doi:10.3389/fnut.2017.00043
[62]. Yeo, Y.Y., Thed, S.T., 2022, Product development of passion fruit and citrus peel dark chocolate. Food Research. 6(S1):41-44. Doi:10.26656/fr.2017.6(s1).010.
Viewed PDF 282 13

