Table of Contents - Issue

Recent articles
-
 Molecular Approach to Identify Anti-inflammatory Potential of Stevioside in HFD-induced Type 2 Diabetic Rats: Evidence From in Vivo StudyAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art001
Molecular Approach to Identify Anti-inflammatory Potential of Stevioside in HFD-induced Type 2 Diabetic Rats: Evidence From in Vivo StudyAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art001Molecular Approach to Identify Anti-inflammatory Potential of Stevioside in HFD-induced Type 2 Diabetic Rats: Evidence From in Vivo Study
Abstract:
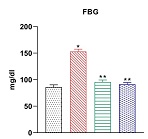
Stevioside is a natural sweetener derived from the leaves of the Stevia rebaudiana plant. It has gained popularity as a sugar substitute due to its intense sweetness without adding calories or affecting blood sugar levels, making it a suitable option for people with diabetes or those looking to reduce their sugar intake. Studies have shown that stevioside has glucose lowering effects. Previous studies have shown that it has significant role in skeletal muscle but its role on expression of inflammatory signaling molecules in adipose tissue against high diet and sucrose-induced type-2 diabetes in experimental rats is yet to be done. The current research was undertaken to investigate if stevioside could also exert its antidiabetic effects by circumventing adipocyte induced inflammation, a key driving factor for insulin resistance in obese individuals. Effective dose of stevioside (20 mg/kg b.wt) was administered orally for 45 days to high fat diet and sucrose induced type-2 diabetic rats. Interestingly, stevioside treatment restores the elevated serum levels of proinflammatory cytokines including tumor necrosis factor-α (TNF-α) and sterol regulatory element binding protein-1c (SREBP-1c) and enhances Peroxisome Proliferator–activated receptor-γ (PPAR-γ) in adipocytes of diabetic rats. The gene expression of IR, GLUT4 and PPAR-γ mRNA were also significantly activated in stevioside treated groups but reduced IL-1 beta, IlL-6, IKKB, TNF-alpha and NFkB mRNA expression in diabetic adipose tissue. More importantly, stevioside acts very effectively as metformin to circumvent inflammation and insulin resistance in diabetic rats. Our results clearly show that stevioside inhibits obesity induced insulin resistance by ameliorating the inflammatory events and upregulating insulin signalling molecules.Keywords: Stevioside, HFD-T2DM, pro inflammation, insulin signalling; adipose tissue, obesity; signaling pathways; Therapautics.Molecular Approach to Identify Anti-inflammatory Potential of Stevioside in HFD-induced Type 2 Diabetic Rats: Evidence From in Vivo Study
References:
[1] DeFronzo, R. A., Ferrannini, E., Groop, L., Henry, R. R., Herman, W. H., Holst, J. J., Hu, F. B., Kahn, C. R., Raz, I., Shulman, G. I., Simonson, D. C., Testa, M. A., & Weiss, R. (2015). Type 2 diabetes mellitus. Nature Reviews Disease Primers, 1(1), 15019. https://doi.org/10.1038/nrdp.2015.19.
[2] Guariguata, L., Whiting, D., Weil, C., & Unwin, N. (2011). The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Research and Clinical Practice, 94(3), 322–332. https://doi.org/10.1016/j.diabres.2011.10.040.
[3] Hu, F. B. (2011). Globalization of Diabetes. Diabetes Care, 34(6), 1249–1257. https://doi.org/10.2337/dc11-0442.
[4] Prasad, M., Rajagopal, P., Devarajan, N., Veeraraghavan. V.P., Palanisamy, C.P., Cui, B., Patil, S., & Jayaraman, S. (2022). A comprehensive review on high -fat diet-induced diabetes mellitus: an epigenetic view. J Nutr Biochem. 107:109037. doi: 10.1016/j.jnutbio.2022.109037.
[5] Kiruthigha, T., Gayathri, R., Vishnu Priya, V., Selvaraj Jayaraman, & Kavitha, S. (2023). Piperine Modulates High Fat Diet - Induced Renal Damage by Regulating Kim-1 and Igf-1 Beta Signaling Molecules in Male Wistar Rats”. Journal of Advanced Zoology, 44 (S5):246-54.
[6] Dandona, P. (2004). Inflammation: the link between insulin resistance, obesity, and diabetes. Trends in Immunology, 25(1), 4–7. https://doi.org/10.1016/j.it.2003.10.013.
[7] Tsalamandris, S., Antonopoulos, A. S., Oikonomou, E., Papamikroulis, G.-A., Vogiatzi, G., Papaioannou, S., Deftereos, S., & Tousoulis, D. (2019). The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. European Cardiology Review, 14(1), 50–59. https://doi.org/10.15420/ecr.2018.33.1.
[8] Cruz, N. G., Sousa, L. P., Sousa, M. O., Pietrani, N. T., Fernandes, A. P., & Gomes, K. B. (2013). The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Research and Clinical Practice, 99(2), 85–92. https://doi.org/10.1016/j.diabres.2012.09.003.
[9] Lontchi-Yimagou, E., Sobngwi, E., Matsha, T. E., & Kengne, A. P. (2013). Diabetes Mellitus and Inflammation. Current Diabetes Reports, 13(3), 435–444. https://doi.org/10.1007/s11892-013-0375-y.
[10] Padmapriya, A., Preetha, S., Selvaraj, J., & Sridevi, G. (2022). Effect of Carica papaya seed extract on IL-6 and TNF-α in human lung cancer cell lines-an In vitro study. Research Journal of Pharmacy and Technology, 15 (12): 5478-5482.
[11] Nath, S., Ghosh, S. K., & Choudhury, Y. (2017). A murine model of type 2 diabetes mellitus developed using a combination of high fat diet and multiple low doses of streptozotocin treatment mimics the metabolic characteristics of type 2 diabetes mellitus in humans. Journal of Pharmacological and Toxicological Methods, 84, 20–30. https://doi.org/10.1016/j.vascn.2016.10.007.
[12] Herieka, M., & Erridge, C. (2014). High‐fat meal induced postprandial inflammation. Molecular Nutrition & Food Research, 58(1), 136–146. https://doi.org/10.1002/mnfr.201300104.
[13] Mounithaa, N., Gayathri, R., Selvaraj Jayaraman, Vishnu Priya, V., & Kavitha, S. (2023). Effect of Piperine on an Nrf2/Keap 1 Signalling Mechanism in Adipose Tissue of High Fat Diet and Sucrose-Induced Experimental Diabetic Rats. Journal of Advanced Zoology, 44 (S5):232-39.
[14] Modak, M., Dixit, P., Londhe, J., Ghaskadbi, S., & Devasagayam, T. P. A. (2007). Indian Herbs and Herbal Drugs Used for the Treatment of Diabetes. Journal of Clinical Biochemistry and Nutrition, 40(3), 163–173. https://doi.org/10.3164/jcbn.40.163.
[15] Pang, G.-M., Li, F.-X., Yan, Y., Zhang, Y., Kong, L.-L., Zhu, P., Wang, K.-F., Zhang, F., Liu, B., & Lu, C. (2019). Herbal medicine in the treatment of patients with type 2 diabetes mellitus. Chinese Medical Journal, 132(1), 78–85. https://doi.org/10.1097/CM9.0000000000000006.
[16] Thana Lakshme, P.S., Gayathri, R., & Vishnu Priya V. (2021). Preliminary Phytochemical Screening and Estimation of Total Phenolic Content of Aqueous Cladode Extract of Opuntia dilleniid. Journal of Research in Medical and Dental Science, 9(2): 254-257.
[17] Mithil Vora, Vishnu Priya, V., Selvaraj,J., Gayathri, R., & Kavitha, S. (2021). Effect of Lupeol on proinflammatory Markers in Adipose Tissue of High-Fat Diet and Sucrose Induced Type-2 Diabetic Rats. Journal of Research in Medical and Dental Science, 9(10):116-121.
[18] Vishaka, S., Sridevi, G., & Selvaraj, J. (2022). An in vitro analysis on the antioxidant and anti-diabetic properties of Kaempferia galanga rhizome using different solvent systems. Journal of Advanced Pharmaceutical Technology and Research, 13 (6): 505-509.
[19] Chen, T.-H., Chen, S.-C., Chan, P., Chu, Y.-L., Yang, H.-Y., & Cheng, J.-T. (2005). Mechanism of the Hypoglycemic Effect of Stevioside, a Glycoside of Stevia rebaudiana. Planta Medica, 71(2), 108–113. https://doi.org/10.1055/s-2005-837775.
[20] Orellana-Paucar, A. M. (2023). Steviol Glycosides from Stevia rebaudiana: An Updated Overview of Their Sweetening Activity, Pharmacological Properties, and Safety Aspects. Molecules, 28(3), 1258. https://doi.org/10.3390/molecules28031258.
[21] Dev Arora, Gayathri, R., Selvaraj, J., Vishnu Priya, V., & Kavitha, S. (2021). Vitamin C and E Down Regulates the Expression of C-JNK, IKKB, NF-kB in Adipose Tissue of PCB-Exposed Rats. Journal of Research in Medical and Dental Science, 9(11):39-44.
[22] Khan, H.L.A., Sridevi, G., Selvaraj, & J. Preetha, S. (2021). In vitro Anti-inflammatory Properties in Various Extracts (Ethanol, Chloroform and Aqueous) of Kaempferia galanga Linn Rhizome. Journal of Pharmaceutical Research International, 33 (47B): 476–481. DOI:https://doi.org/10.9734/jpri/2021/v33i47B33146.
[23] Ponnulakshmi, R., Shyamaladevi, B., Vijayalakshmi, P., & Selvaraj, J. (2019). In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicology mechanisms and methods, 29(4), 276–290. https://doi.org/10.1080/15376516.2018.1545815.
[24] Jayaraman, S., Devarajan, N., Rajagopal, P., Babu, S., Ganesan, S.K., Veeraraghavan, V.P., Palanisamy, C.P., Cui, B., Periyasamy, V., & Chandrasekar K. (2021). β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules. 26(7), 2101. doi: 10.3390/molecules26072101.
[25] Fan, C., Song, Q., Wang, P., Li, Y., Yang, M., & Yu, S.Y. (2019). Neuroprotective Effects of Curcumin on IL-1β-Induced Neuronal Apoptosis and Depression-Like Behaviors Caused by Chronic Stress in Rats. Frontiers in Cellular Neuroscience, 7, 12:516. doi: 10.3389/fncel.2018.00516.
[26] Zhang, M., Lv, X.-Y., Li, J., Xu, Z.-G., & Chen, L. (2008). The Characterization of High-Fat Diet and Multiple Low-Dose Streptozotocin Induced Type 2 Diabetes Rat Model. Experimental Diabetes Research, 2008, 1–9. https://doi.org/10.1155/2008/704045.
[27] Nabi, S. A., Kasetti, R. B., Sirasanagandla, S., Tilak, T. K., Kumar, M. V. J., & Rao, C. A. (2013). Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Complementary and Alternative Medicine, 13(1), 37. https://doi.org/10.1186/1472-6882-13-37.
[28] Jayaraman, S., Krishnamoorthy, K., Prasad, M., Veeraraghavan, V.P., Krishnamoorthy, R., Alshuniaber, M.A., Gatasheh, M.K., Elrobh, & M., Gunassekaran. (2023). Glyphosate potentiates insulin resistance in skeletal muscle through the modulation of IRS-1/PI3K/Akt mediated mechanisms: An in vivo and in silico analysis. Int J Biol Macromol, 242(Pt 2):124917. doi: 10.1016/j.ijbiomac.2023.124917.
[29] Prasad, M., Jayaraman, S., Natarajan, S.R., Veeraraghavan, V.P., Krishnamoorthy, R., Gatasheh, M.K., Palanisamy, C.P., & Elrobh, M. (2023). Piperine modulates IR/Akt/GLUT4 pathways to mitigate insulin resistance: Evidence from animal and computational studies. Int J Biol Macromol, 253(Pt 5):127242. doi: 10.1016/j.ijbiomac.2023.127242.
[30] Shen, P., Liu, M., Ng, T., Chan, Y., & Yong, E. L. (2006). Differential Effects of Isoflavones, from Astragalus Membranaceus and Pueraria Thomsonii, on the Activation of PPARα, PPARγ, and Adipocyte Differentiation In Vitro. The Journal of Nutrition, 136(4), 899–905. https://doi.org/10.1093/jn/136.4.899.
[31] Akifa Begum, Palati Sinduja, Priyadharshini, R., & Selvaraj Jayaraman. (2021). Estimation of Clinocopathological Correlation and Comparison of Salivary TNF-α among Normal and Post Radiotherapy Patients of Oral cancer-A Cross-Sectional Study. Journal of Research in Medical and Dental Science, 9(10): 92-97.
[32] Fathima Hinaz, Z., Gayathri, R., Selvaraj, J., Vishnu Priya, V., Kavitha, S., & Gayathri, R. (2021). Comparative Evaluation of Anti-Cholesterol Potential of Apple Cider Vinegar and Its Herbal Formulation with Allium Sativum and Honey-An In-Vitro Assay. Journal of Research in Medical and Dental Science 9 (10),142-147.
[33] Picard, F., & Auwerx, J. (2002). PPARγ and glucose homeostasis. Annual Review of Nutrition, 22(1), 167–197. https://doi.org/10.1146/annurev.nutr.22.010402.102808.
[34] Staels, B., & Fruchart, J.-C. (2005). Therapeutic Roles of Peroxisome Proliferator–Activated Receptor Agonists. Diabetes, 54(8), 2460–2470. https://doi.org/10.2337/diabetes.54.8.2460.
[35] Daynes, R. A., & Jones, D. C. (2002). Emerging roles of PPARS in inflammation and immunity. Nature Reviews Immunology, 2(10), 748–759. https://doi.org/10.1038/nri912.
[36] Sharma, B., Salunke, R., Srivastava, S., Majumder, C., & Roy, P. (2009). Effects of guggulsterone isolated from Commiphora mukul in high fat diet induced diabetic rats. Food and Chemical Toxicology, 47(10), 2631–2639. https://doi.org/10.1016/j.fct.2009.07.021.
Viewed PDF 917 30 -
 Sativoside Mitigates High-Fat Diet-Induced Inflammation and Type-2 Diabetes in Adipose Tissue of Wistar RatsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art002
Sativoside Mitigates High-Fat Diet-Induced Inflammation and Type-2 Diabetes in Adipose Tissue of Wistar RatsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art002Sativoside Mitigates High-Fat Diet-Induced Inflammation and Type-2 Diabetes in Adipose Tissue of Wistar Rats
Abstract:
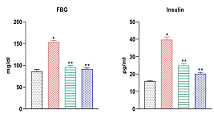
This study aimed to investigate the impact of Stevioside, on the biochemical changes in high-fat diet-fed Wistar rats. Adult male Wistar rats were induced into a diabetic state through the administration of a high-fat diet and sucrose for 60 days, followed by oral administration of stevioside (20 mg/kg/day) for 45 days. Various parameters, including fasting blood glucose, oral glucose tolerance, insulin, insulin tolerance, liver function (ALT, AST, ALP), kidney function (urea and creatinine), and lipid profiles (TC, TG, FFA, HDL-c and LDL-c), serum adipokines levels such as adiponectin, leptin, resistin were assessed. Stevioside treatment notably improved glucose and insulin tolerances in diabetic rats and normalized their elevated levels of fasting blood glucose, serum insulin, and lipid profile. In the high-fat diet-induced type 2 diabetes rat model, Stevioside effectively restored the altered blood serum levels, demonstrating efficacy comparable to that of metformin. Therefore, Stevioside displays promise as a potential phytomedicine for managing type 2 diabetes mellitus.Keywords: High-fat diet, Insulin tolerance, Type-2 diabetes, Stevia rebaudiana.Sativoside Mitigates High-Fat Diet-Induced Inflammation and Type-2 Diabetes in Adipose Tissue of Wistar Rats
References:
[1] Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant. 2011 Jan;26(1):28-35. doi: 10.1093/ndt/gfq576. Epub 2010 Nov 2. PMID: 21045078.
[2] Xu G, Liu B, Sun Y, Du Y, Snetselaar LG, Hu FB, Bao W. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018 Sep 4;362:k1497. doi: 10.1136/bmj.k1497. PMID: 30181166; PMCID: PMC6122253.
[3] Prasad M, Rajagopal P, Devarajan N, Veeraraghavan VP, Palanisamy CP, Cui B, Patil S, Jayaraman S. A comprehensive review on high -fat diet-induced diabetes mellitus: an epigenetic view. J Nutr Biochem. 2022 Sep;107:109037. doi: 10.1016/j.jnutbio.2022.109037. Epub 2022 May 6. PMID: 35533900.
[4] Prasad M, Jayaraman S, Natarajan SR, Veeraraghavan VP, Krishnamoorthy R, Gatasheh MK, Palanisamy CP, Elrobh M. Piperine modulates IR/Akt/GLUT4 pathways to mitigate insulin resistance: Evidence from animal and computational studies. Int J Biol Macromol. 2023 Dec 31;253(Pt 5):127242. doi: 10.1016/j.ijbiomac.2023.127242. Epub 2023 Oct 4. PMID: 37797864.
[5] Kiruthigha T, Gayathri R, Vishnu Priya V, Selvaraj J, Kavitha, S. Piperine Modulates High Fat Diet - Induced Renal Damage by Regulating Kim-1 and Igf-1 Beta Signaling Molecules in Male Wistar Rats”. J. Adv. Zool. 2023 44 (S5):246-54.
[6] Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, Nichols G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010 Mar;87(3):293-301. doi: 10.1016/j.diabres.2010.01.026. Epub 2010 Feb 19. Erratum in: Diabetes Res Clin Pract. 2011 May;92(2):301. PMID: 20171754.
[7] Lozano I, Van der Werf R, Bietiger W, Seyfritz E, Peronet C, Pinget M, Jeandidier N, Maillard E, Marchioni E, Sigrist S, Dal S. High-fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutr Metab (Lond). 2016 Feb 25;13:15. doi: 10.1186/s12986-016-0074-1. PMID: 26918024; PMCID: PMC4766713.
[8] Goyal SK, Samsher, Goyal RK. Stevia (Stevia rebaudiana) a bio-sweetener: a review. Int J Food Sci Nutr. 2010 Feb;61(1):1-10. doi: 10.3109/09637480903193049. PMID: 19961353.
[9] Samuel P, Ayoob KT, Magnuson BA, Wölwer-Rieck U, Jeppesen PB, Rogers PJ, Rowland I, Mathews R. Stevia Leaf to Stevia Sweetener: Exploring Its Science, Benefits, and Future Potential. J Nutr. 2018 Jul 1;148(7):1186S-1205S. doi: 10.1093/jn/nxy102. PMID: 29982648.
[10] Orellana-Paucar AM. Steviol Glycosides from Stevia rebaudiana: An Updated Overview of Their Sweetening Activity, Pharmacological Properties, and Safety Aspects. Molecules. 2023 Jan 27;28(3):1258. doi: 10.3390/molecules28031258. PMID: 36770924; PMCID: PMC9920402.
[11] Barriocanal LA, Palacios M, Benitez G, Benitez S, Jimenez JT, Jimenez N, Rojas V. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans. A pilot study of repeated exposures in some normotensive and hypotensive individuals and in Type 1 and Type 2 diabetics. Regul Toxicol Pharmacol. 2008 Jun;51(1):37-41. doi: 10.1016/j.yrtph.2008.02.006. Epub 2008 Mar 5. PMID: 18397817.
[12] Carrera-Lanestosa A, Moguel-Ordóñez Y, Segura-Campos M. Stevia rebaudiana Bertoni: A Natural Alternative for Treating Diseases Associated with Metabolic Syndrome. J Med Food. 2017 Oct;20(10):933-943. doi: 10.1089/jmf.2016.0171. Epub 2017 Aug 9. PMID: 28792778; PMCID: PMC5651958.
[13] Ruiz-Ruiz JC, Moguel-Ordoñez YB, Segura-Campos MR. Biological activity of Stevia rebaudiana Bertoni and their relationship to health. Crit Rev Food Sci Nutr. 2017 Aug 13;57(12):2680-2690. doi: 10.1080/10408398.2015.1072083. PMID: 26479769.
[14] Lemus-Mondaca R, Vega-Gálvez A, Zura-Bravo L, Ah-Hen K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional, and functional aspects. Food Chem. 2012 Jun 1;132(3):1121-1132. doi: 10.1016/j.foodchem.2011.11.140. Epub 2011 Dec 13. PMID: 29243591.
[15] Thana Lakshme, P.S., Gayathri, R., Vishnu Priya V. Preliminary Phytochemical Screening and Estimation of Total Phenolic Content of Aqueous Cladode Extract of Opuntia dilleniid. J. Res. Med. Dent. Sci. 2021 9(2): 254-257.
[16] Mithil Vora, Vishnu Priya V, Selvaraj J, Gayathri R, Kavitha S. Effect of Lupeol on proinflammatory Markers in Adipose Tissue of High-Fat Diet and Sucrose Induced Type-2 Diabetic Rats. J. Res. Med. Dent. Sci. 2021 9(10):116-121.
[17] Vishaka S, Sridevi G, Selvaraj J. An in vitro analysis on the antioxidant and anti-diabetic properties of Kaempferia galanga rhizome using different solvent systems. J Adv Pharm Technol Res. 2022 Dec;13(Suppl 2):S505-S509. doi: 10.4103/japtr.japtr_189_22. Epub 2022 Dec 30. PMID: 36798576; PMCID: PMC9926592.
[18] Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 2014 Jul;5(4):349-58. doi: 10.1111/jdi.12235. Epub 2014 May 19. PMID: 25411593; PMCID: PMC4210077.
[19] Holmes A, Coppey LJ, Davidson EP, Yorek MA. Rat Models of Diet-Induced Obesity and High Fat/Low Dose Streptozotocin Type 2 Diabetes: Effect of Reversal of High Fat Diet Compared to Treatment with Enalapril or Menhaden Oil on Glucose Utilization and Neuropathic Endpoints. J Diabetes Res. 2015;2015:307285. doi: 10.1155/2015/307285. Epub 2015 Jul 2. PMID: 26229968.
[20] Dev Arora, Gayathri R, Selvaraj J, Vishnu Priya V, Kavitha S. Vitamin C and E Down Regulates the Expression of C-JNK, IKKB, NF-kB in Adipose Tissue of PCB-Exposed Rats. J. Res. Med. Dent. Sci. 2021 9(11):39-44.
[21] Khan, HLA, Sridevi G, Selvaraj J, Preetha S. In vitro Anti-inflammatory Properties in Various Extracts (Ethanol, Chloroform and Aqueous) of Kaempferia galanga Linn Rhizome. J. Pharm. Res. Int. 2021 33 (47B): 476–481. DOI:https://doi.org/10.9734/jpri/2021/v33i47B33146.
[22] McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab. 2009 Oct;297(4):E849-55. doi: 10.1152/ajpendo.90996.2008. Epub 2009 Jul 28. PMID: 19638507; PMCID: PMC2763792.
[23] Nagy C, Einwallner E. Study of In Vivo Glucose Metabolism in High-fat Diet-fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J Vis Exp. 2018 Jan 7;(131):56672. doi: 10.3791/56672. PMID: 29364280; PMCID: PMC5908452.
[24] Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008 Dec;295(6):E1323-32. doi: 10.1152/ajpendo.90617.2008. Epub 2008 Sep 23. PMID: 18812462.
[25] Akifa Begum, Palati Sinduja, Priyadharshini R, Selvaraj Jayaraman. Estimation of Clinocopathological Correlation and Comparison of Salivary TNF-α among Normal and Post Radiotherapy Patients of Oral cancer-A Cross-Sectional Study. J. Res. Med. Dent. Sci. 2021 9(10): 92-97.
[26] Fathima Hinaz Z, Gayathri R, Selvaraj J, Vishnu Priya V, Kavitha, S, Gayathri R. Comparative Evaluation of Anti-Cholesterol Potential of Apple Cider Vinegar and Its Herbal Formulation with Allium Sativum and Honey-An In-Vitro Assay. J. Res. Med. Dent. Sci. 2021 9 (10),142-147.
[27] Logan IE, Bobe G, Miranda CL, Vasquez-Perez S, Choi J, Lowry MB, Sharpton TJ, Morgun A, Maier CS, Stevens JF, Shulzhenko N, Gombart AF. Germ-Free Swiss Webster Mice on a High-Fat Diet Develop Obesity, Hyperglycemia, and Dyslipidemia. Microorganisms. 2020 Apr 5;8(4):520. doi: 10.3390/microorganisms8040520. PMID: 32260528; PMCID: PMC7232377.
[28] Rotimi SO, Rotimi OA, Adelani IB, Onuzulu C, Obi P, Okungbaye R. Stevioside modulates oxidative damage in the liver and kidney of high fat/low streptozocin diabetic rats. Heliyon. 2018 May 31;4(5):e00640. doi: 10.1016/j.heliyon.2018.e00640. PMID: 29872771; PMCID: PMC5986550.
[29] Mounithaa N, Gayathri R, Selvaraj Jayaraman, Vishnu Priya V, Kavitha S. Effect of Piperine on an Nrf2/Keap 1 Signalling Mechanism in Adipose Tissue of High Fat Diet and Sucrose-Induced Experimental Diabetic Rats. J. Adv. Zool. 2023 44 (S5):232-39.
[30] Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med. 2019 Jul;44(1):3-15. doi: 10.3892/ijmm.2019.4188. Epub 2019 May 8. PMID: 31115493; PMCID: PMC6559295.
[31] Padmapriya, A., Preetha, S., Selvaraj, J., Sridevi, G. (2022). Effect of Carica papaya seed extract on IL-6 and TNF-α in human lung cancer cell lines-an In vitro study. Res J Pharm Technol. 2022 15 (12): 5478-5482.
[32] Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010 Nov;1212:E1-E19. doi: 10.1111/j.1749-6632.2010.05875.x. Erratum in: Ann N Y Acad Sci. 2011 May;1226(1):50. PMID: 21276002; PMCID: PMC3075414.
[33] Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne). 2013 Jun 12;4:71. doi: 10.3389/fendo.2013.00071. PMID: 23781214; PMCID: PMC3679475.
[34] Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014 Apr 11;15(4):6184-223. doi: 10.3390/ijms15046184. PMID: 24733068; PMCID: PMC4013623.
[35] Deenadayalan A, Subramanian V, Paramasivan V, Veeraraghavan VP, Rengasamy G, Coiambatore Sadagopan J, Rajagopal P, Jayaraman S. Stevioside Attenuates Insulin Resistance in Skeletal Muscle by Facilitating IR/IRS-1/Akt/GLUT 4 Signaling Pathways: An In Vivo and In Silico Approach. Molecules. 2021 Dec 20;26(24):7689. doi: 10.3390/molecules26247689. PMID: 34946771; PMCID: PMC8707280.
[36] Jayaraman S, Krishnamoorthy K, Prasad M, Veeraraghavan VP, Krishnamoorthy R, Alshuniaber MA, Gatasheh MK, Elrobh M, Gunassekaran. Glyphosate potentiates insulin resistance in skeletal muscle through the modulation of IRS-1/PI3K/Akt mediated mechanisms: An in vivo and in silico analysis. Int J Biol Macromol. 2023 Jul 1;242(Pt 2):124917. doi: 10.1016/j.ijbiomac.2023.124917. Epub 2023 May 18. PMID: 37207753.
Viewed PDF 889 13 -
 Synthesis, Characterisation, and in Vitro Biocompatibility Studies of Selenium Nanoparticles Synthesized using Hybanthus Enneaspermus Plant Extract for Potential Biomedical ApplicationsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art003
Synthesis, Characterisation, and in Vitro Biocompatibility Studies of Selenium Nanoparticles Synthesized using Hybanthus Enneaspermus Plant Extract for Potential Biomedical ApplicationsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art003Synthesis, Characterisation, and in Vitro Biocompatibility Studies of Selenium Nanoparticles Synthesized using Hybanthus Enneaspermus Plant Extract for Potential Biomedical Applications
Abstract:
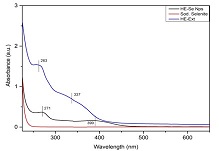
Hybanthus enneaspermus (HE) is a traditional medicinal plant used for treating various disease conditions. Selenium nanoparticles (SeNPs) possess various properties such as anticancer, antioxidant, etc. The objective of the present study is to conduct green synthesis of selenium nanoparticles using Hybanthus enneaspermus(HE) and evaluate their biocompatibility. Leaves of HE are utilized for synthesizing SeNPs. Characterization studies of HE-SeNPs are carried out using UV spectrophotometry, FT-IR spectroscopy, and SEM. To check the biocompatibility, hemolytic assay, and Annexin V-PI assays are carried out. A change in color is observed after the addition of sodium selenite to the leaf extract. UV spectrophotometry gives a peak at 271 nm confirming the synthesis of SeNPs. FT-IR gives peaks at 3224, 1565, 1399, 1078, 784, and 717 cm-1 with a fingerprint of 3500 - 1000 cm-1. SEM analysis shows the spherical morphology of the SeNPs. HE-SeNPs at lower concentrations cause less hemolysis. However, HE-SeNPs are found to be less biocompatible, so further studies are needed to confirm their biocompatible nature. SeNPs synthesized from HE can be ideal for biomedical applications but further studies are required to check its biocompatibility.
Keywords: SeNPs, Hybanthus enneaspermus, green synthesis, biocompatibility.Synthesis, Characterisation, and in Vitro Biocompatibility Studies of Selenium Nanoparticles Synthesized using Hybanthus Enneaspermus Plant Extract for Potential Biomedical Applications
References:
[1] Wacker, M. G. (2014). Nanotherapeutics—Product Development Along the “Nanomaterial” Discussion. Journal of pharmaceutical sciences, 103(3), 777–784. https://doi.org/10.1002/jps.23879.
[2] Abbasian, R., & Jafarizadeh-Malmiri, H. (2020). Green approach in gold, silver and selenium nanoparticles using coffee bean extract. Open Agriculture, 5(1), 761–767. https://doi.org/10.1515/opag-2020-0074.
[3] Fardsadegh, B., & Jafarizadeh-Malmiri, H. (2019). Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Processing and Synthesis, 8(1), 399–407. https://doi.org/10.1515/gps-2019-0007.
[4] Nasim, I., Rajeshkumar, S., & Vishnupriya, V. (2021). Green synthesis of reduced graphene oxide nanoparticles, its characterization, and antimicrobial properties against common oral pathogens. Int J Dentistry Oral Sci., 8(2), 1670–1675.
[5] Nasim, I., Rajesh Kumar, S., Vishnupriya, V., & Jabin, Z. (2020). Cytotoxicity and anti-microbial analysis of silver and graphene oxide bio nanoparticles. Bioinformation, 16(11), 831–836. https://doi.org/10.6026/97320630016831.
[6] Fritea, L., Laslo, V., Cavalu, S., Costea, T., & Vicas, S. I. (2017). Green biosynthesis of selenium nanoparticles using parsley (Petroselinum crispum) leaves extract. Studia Universitatis” Vasile Goldis” Arad. Seria Stiintele Vietii (Life Sciences Series), 27(3), 203–208. Retrieved from http://www.studiauniversitatis.ro/pdf/27-%202017/27-3-2017/7-%20SUVG-27-3-%20L.F.-%20203-208.pdf.
[7] Kishore, S. O. G., Priya, A. J., & Narayanan, L. (n.d.). Controlling of oral pathogens using turmeric and tulsi herbal formulation mediated copper nanoparticles. Plant cell biotechnology and molecular biology.
[8] Rieshy, V., Priya, J., Arivarasu, L., & Kumar, S. R. (n.d.). Enhanced Antimicrobial Activity of Herbal Formulation Mediated Copper Nanoparticles Against Clinical Pathogens. The Plant cell.
[9] Rajeshkumar, S., & Lakshmi, S. (2021). Anticariogenic activity of silver nanoparticles synthesized using fresh leaves extract of kalanchoe pinnata. . Int J Dentistry Oral Sci., 8(7), 2985–2987.
[10] Rajeshkumar, S., Lakshmi, T., & Tharani, M. (2021). Green synthesis of copper nanoparticles synthesized using black tea and its antibacterial activity against oral pathogens. Int. J. Dent. Oral Sci., 8(9), 4156–4159.
[11] Maheswari, T. N. U., & Dhanvanth, M. (2022). Topical herbal therapeutic formulation used in the management of oral potentially malignant disorders – A systematic review. Journal of Indian Academy of Oral Medicine and Radiology, 34(2), 223. https://doi.org/10.4103/jiaomr.jiaomr_101_21.
[12] Radomska, D., Czarnomysy, R., Radomski, D., Bielawska, A., & Bielawski, K. (2021). Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients, 13(5). https://doi.org/10.3390/nu13051649
[13] Souza, L. M. dos S., Dibo, M., Sarmiento, J. J. P., Seabra, A. B., Medeiros, L. P., Lourenço, I. M., … Nakazato, G. (2022). Biosynthesis of selenium nanoparticles using combinations of plant extracts and their antibacterial activity. Current Research in Green and Sustainable Chemistry, 5, 100303. https://doi.org/10.1016/j.crgsc.2022.100303.
[14] Johnson, J., Shanmugam, R., & Lakshmi, T. (2022). A review on plant-mediated selenium nanoparticles and its applications. Journal of population therapeutics and clinical pharmacology = Journal de la therapeutique des populations et de la pharamcologie clinique, 28(2), e29–e40. https://doi.org/10.47750/jptcp.2022.870.
[15] Mi, X.-J., Choi, H. S., Perumalsamy, H., Shanmugam, R., Thangavelu, L., Balusamy, S. R., & Kim, Y.-J. (2022). Biosynthesis and cytotoxic effect of silymarin-functionalized selenium nanoparticles induced autophagy mediated cellular apoptosis via downregulation of PI3K/Akt/mTOR pathway in gastric cancer. Phytomedicine: international journal of phytotherapy and phytopharmacology, 99, 154014. https://doi.org/10.1016/j.phymed.2022.154014.
[16] Sneka, & Santhakumar, P. (2021). Antibacterial Activity of Selenium Nanoparticles extracted from Capparis decidua against Escherichia coli and Lactobacillus Species. Journal of advanced pharmaceutical technology & research, 14(8), 4452–4454. https://doi.org/10.52711/0974-360x.2021.00773.
[17] Pandiyan, I., Sri, S. D., Indiran, M. A., Rathinavelu, P. K., Prabakar, J., & Rajeshkumar, S. (2022). Antioxidant, anti-inflammatory activity of Thymus vulgaris-mediated selenium nanoparticles: An in vitro study. Journal of conservative dentistry: JCD, 25(3), 241–245. https://doi.org/10.4103/JCD.JCD_369_21.
[18] Hosnedlova, B., Kepinska, M., Skalickova, S., Fernandez, C., Ruttkay-Nedecky, B., Peng, Q., … Kizek, R. (2018). Nano-selenium and its nanomedicine applications: a critical review. International journal of nanomedicine, 13, 2107–2128. https://doi.org/10.2147/IJN.S157541.
[19] Benstoem, C., Goetzenich, A., Kraemer, S., Borosch, S., Manzanares, W., Hardy, G., & Stoppe, C. (2015). Selenium and its supplementation in cardiovascular disease--what do we know? Nutrients, 7(5), 3094–3118. https://doi.org/10.3390/nu7053094.
[20] Kamath, K. A., Nasim, I., & Rajeshkumar, S. (2020). Evaluation of the re-mineralization capacity of a gold nanoparticle-based dental varnish: An in vitro study. Journal of conservative dentistry: JCD, 23(4), 390–394.
[21] Nasim, I., Jabin, Z., Kumar, S. R., & Vishnupriya, V. (2022). Green synthesis of calcium hydroxide-coated silver nanoparticles using Andrographis paniculata and Ocimum sanctum Linn. leaf extracts: An antimicrobial and cytotoxic activity. Journal of conservative dentistry: JCD, 25(4), 369–374. https://doi.org/10.4103/jcd.jcd_411_21.
[22] Wadhwani, S. A., Gorain, M., Banerjee, P., Shedbalkar, U. U., Singh, R., Kundu, G. C., & Chopade, B. A. (2017). Green synthesis of selenium nanoparticles using Acinetobacter sp. SW30: optimization, characterization and its anticancer activity in breast cancer cells. International journal of nanomedicine, 12, 6841–6855. https://doi.org/10.2147/IJN.S139212.
[23] Faramarzi, S., Anzabi, Y., & Jafarizadeh-Malmiri, H. (2020). Nanobiotechnology approach in intracellular selenium nanoparticle synthesis using Saccharomyces cerevisiae—fabrication and characterization. Archives of microbiology, 202(5), 1203–1209. https://doi.org/10.1007/s00203-020-01831-0.
[24] Maliael, M. T., Jain, R. K., & Srirengalakshmi, M. (2022). Effect of nanoparticle coatings on frictional resistance of orthodontic archwires: a systematic review and meta-analysis. World J. Dent, 13(4), 417–424. Retrieved from https://www.wjoud.com/abstractArticleContentBrowse/WJOUD/28479/JPJ/fullText.
[25] Sushanthi, S., Srisakthi, D., MeignanaArumugham, I., Pradeepkumar, R., & Rajeshkumar, S. (2021). Vernonia Amygdalina Mediated Copper Nanoparticles and its Characterization and Antimicrobial Activity - An In Vitro Study. Int J Dentistry Oral Sci., 8(7), 3330–3334.
[26] Shekhawat, M. S., & Manokari, M. (2018). In vitro multiplication, micromorphological studies and ex vitro rooting of Hybanthus enneaspermus (L.) F. Muell. – a rare medicinal plant. Acta Botanica Croatica. https://doi.org/10.1515/botcro-2017-0012.
[27] Patel, D. K., Kumar, R., Laloo, D., & Hemalatha, S. (2011). Evaluation of phytochemical and antioxidant activities of the different fractions of Hybanthus enneaspermus (Linn.) F. Muell. (Violaceae). Asian Pacific journal of tropical medicine, 4(5), 391–396. https://doi.org/10.1016/S1995-7645(11)60110-7.
[28] Patel, D. K., Kumar, R., Prasad, S. K., Sairam, K., & Hemalatha, S. (2011). Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin-induced diabetic rats. Asian Pacific journal of tropical biomedicine, 1(4), 316–322. https://doi.org/10.1016/S2221-1691(11)60051-8.
[29] Patel, D. K., Kumar, R., Sairam, K., & Hemalatha, S. (2013). Hybanthus enneaspermus (L.) F. Muell: a concise report on its phytopharmacological aspects. Chinese journal of natural medicines, 11(3), 199–206. https://doi.org/10.1016/S1875-5364(13)60017-5.
[30] Tripathy, S., Sahoo, S. P., Pradhan, D., Sahoo, S., & Satapathy, D. K. (2009). Evaluation of anti arthritic potential of Hybanthus enneaspermus. Retrieved February 6, 2023, from https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=b493b471da54de812289586c9fe53948b45e25f6.
[31] Anand, T., & Gokulakrishnan, K. (2012). Phytochemical analysis of Hybanthus enneaspermus using UV, FTIR and GC-MS. IOSR Journal of Pharmacy, 2(3), 520–524. Retrieved from https://www.researchgate.net/profile/Anand_Thirupathi2/publication/266286276_Phytochemical_analysis_of_hybanthus_enneaspermus_using_UV_FTIR_and_GC-_MS/links/55808d8808ae47061e5f3311.pdf.
[32] Rajsekhar, P. B., Bharani, R. S. A., Angel, K. J., Ramachandran, M., & Rajsekhar, S. P. V. (2016). Hybanthus enneaspermus (L) F. Muell: A phytopharmacological review on herbal medicine. Journal of chemical and pharmaceutical research, 8(1), 351–355.
[33] Hemalatha, S., Wahi, A. K., Singh, P. N., & Chansouria, J. P. N. (2003). Anticonvulsant and free radical scavenging activity of Hybanthus enneaspermus: A preliminary screening. Indian journal of traditional knowledge. Retrieved from http://nopr.niscpr.res.in/handle/123456789/25972.
[34] Weniger, B., Lagnika, L., Vonthron-Sénécheau, C., Adjobimey, T., Gbenou, J., Moudachirou, M., … Sanni, A. (2004). Evaluation of ethnobotanically selected Benin medicinal plants for their in vitro antiplasmodial activity. Journal of ethnopharmacology, 90(2-3), 279–284. https://doi.org/10.1016/j.jep.2003.10.002.
[35] Francis, A. P., Gurudevan, S., & Jayakrishnan, A. (2018). Synthetic polymannose as a drug carrier: synthesis, toxicity and anti-fungal activity of polymannose-amphotericin B conjugates. Journal of biomaterials science. Polymer edition, 29(13), 1529–1548. https://doi.org/10.1080/09205063.2018.1469186.
[36] Anu, K., Singaravelu, G., Murugan, K., & Benelli, G. (2017). Green-Synthesis of Selenium Nanoparticles Using Garlic Cloves (Allium sativum): Biophysical Characterization and Cytotoxicity on Vero Cells. Journal of Cluster Science, 28(1), 551–563. https://doi.org/10.1007/s10876-016-1123-7.
[37] Alagesan, V., & Venugopal, S. (2019). Green Synthesis of Selenium Nanoparticle Using Leaves Extract of Withania somnifera and Its Biological Applications and Photocatalytic Activities. BioNanoScience, 9(1), 105–116. https://doi.org/10.1007/s12668-018-0566-8.
Viewed PDF 1139 30 -
 One Pot Synthesis of Colloidal Zirconium Nanoparticles using Orthosiphon Stamineus Leaf Extract for Potential Bone Tissue Engineering ApplicationsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art004
One Pot Synthesis of Colloidal Zirconium Nanoparticles using Orthosiphon Stamineus Leaf Extract for Potential Bone Tissue Engineering ApplicationsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art004One Pot Synthesis of Colloidal Zirconium Nanoparticles using Orthosiphon Stamineus Leaf Extract for Potential Bone Tissue Engineering Applications
Abstract:
Zirconium (ZrO2) is a metal oxide nanoparticles (NPs) possessing antimicrobial, antifungal, antioxidant, biosensing, biocompatibility, and anticancer activities. Due to their unique properties, ZrNPs can be used for multiple biomedical applications. Orthosiphon stamineus (OS) is a perennial medicinal herb with potent bioactive constituents. Traditionally, it was used in treating rheumatism, epilepsy, jaundice, hepatitis, etc, Hence OS could be used as a capping agent for synthesizing ZrO2NPs. The current study aimed to synthesize ZrO2NPs using a green source like Orthosiphon stamineus(OS) leaves extract and characterized using UV spectrophotometry, FTIR, SEM, EDX, and their biocompatibility was tested using Annexin V apoptosis assay. Milky precipitation formed, followed by the addition of OS extract to the aqueous solution of Zirconium oxychloride Octahydrate, revealed the formation of ZrO2NPs was further confirmed by the maximum absorbance at 296 nm in the UV-Vis spectrum. The peaks in the fingerprint region of FTIR revealed the presence of the functional groups of the phytoconstituents, confirming the capping. Apoptosis assay revealing the strong biocompatibility of ZrO2NPs towards peripheral blood mononuclear cells with 77.64% cell viability. From the apoptosis assay, it was evident that ZrO2NPs are less cytotoxic, indicating their applicability for medicinal applications. However further studies are required to validate its actions on bone tissue engineering.
Keywords: Green synthesis, Leaf extract, Nanoparticles, Cytotoxicity.One Pot Synthesis of Colloidal Zirconium Nanoparticles using Orthosiphon Stamineus Leaf Extract for Potential Bone Tissue Engineering Applications
References:
[1] Surendiran, A., Sandhiya, S., Pradhan, S. C., and Adithan, C. 2009. Novel applications of nanotechnology in medicine. Indian J. Med. Res. 130 (6): 689–701.
[2] Annu, A., Sivasankari, C., and Krupasankar, U. 2020. Synthesis and characterization of ZrO2 nanoparticles by leaf extract bioreduction process for its biological studies. Mater. Today: Proc. 33 (80): 5317-5323.
[3] Van Tran, T., Nguyen, D. T. C., Kumar, P. S., Din, A. T. M., Jalil, A. A., and Vo, D.-V. N. 2022. Green synthesis of ZrO2 nanoparticles and nanocomposites for biomedical and environmental applications: a review. Environ. Chem. Lett. 20: 1309–1331.
[4] Shojaei, T. R., Soltani, S., and Derakhshani, M. 2022. Synthesis, properties, and biomedical applications of inorganic bionanomaterials. In: Fundamentals of Bionanomaterials: Micro and Nano Technologies, Barhoum, A., Jeevanandam, J., and Danquah, M.K., Elsevier, Amsterdam, Netherlands pp: 139-174.
[5] Chau, T. P., Veeraragavan, G. R., Narayanan, M., Chinnathambi, A., Alharbi, S. A., Subramani, B., … Pikulkaew, S. 2022. Green synthesis of Zirconium nanoparticles using Punica granatum (pomegranate) peel extract and their antimicrobial and antioxidant potency. Environ. Res. 209: 112771.
[6] Ying, S., Guan, Z., Ofoegbu, P. C., Clubb, P., Rico, C., He, F., and Hong, J. 2022. Green synthesis of nanoparticles: Current developments and limitations. Environmental Technology & Innovation. https://doi.org/10.1016/j.eti.2022.102336
[7] Johnson, J., Shanmugam, R., and Lakshmi, T. 2022. A review on plant-mediated selenium nanoparticles and its applications. J. Popul. Ther. Clin. Pharmacol. 28 (2): e29–e40.
[8] Liang, Y., Demir, H., Wu, Y., Aygun, A., Elhouda Tiri, R. N., Gur, T., … Vasseghian, Y. 2022. Facile synthesis of biogenic palladium nanoparticles using biomass strategy and application as photocatalyst degradation for textile dye pollutants and their in-vitro antimicrobial activity. Chemosphere, 306: 135518.
[9] Pandiyan, I., Sri, S. D., Indiran, M. A., Rathinavelu, P. K., Prabakar, J., and Rajeshkumar, S. 2022. Antioxidant, anti-inflammatory activity of Thymus vulgaris-mediated selenium nanoparticles: An in vitro study. J. Conserv. Dent. 25 (3): 241–245.
[10] Sushanthi, S., Srisakthi, D., Meignana Arumugham, I., Pradeepkumar, R., and Rajeshkumar, S. 2021. Vernonia Amygdalina Mediated Copper Nanoparticles and its Characterization and Antimicrobial Activity - An In Vitro Study. Int J Dentistry Oral Sci., 8 (7): 3330–3334.
[11] Qamar, S. U. R., and Ahmad, J. N. 2021. Nanoparticles: Biosynthesis mechanism using plant extracts, bacteria, fungi, and their applications. J. Mol. Liq. 334: 116040
[12] Ameer, O. Z., Salman, I. M., Asmawi, M. Z., Ibraheem, Z. O., and Yam, M. F. 2012. Orthosiphon stamineus: traditional uses, phytochemistry, pharmacology, and toxicology. J. Med. Food, 15 (8): 678–690.
[13] Mi, X.-J., Choi, H. S., Perumalsamy, H., Shanmugam, R., Thangavelu, L., Balusamy, S. R., and Kim, Y.-J. 2022. Biosynthesis and cytotoxic effect of silymarin-functionalized selenium nanoparticles induced autophagy mediated cellular apoptosis via downregulation of PI3K/Akt/mTOR pathway in gastric cancer. Phytomedicine, 99: 154014.
[14] Maliael, M. T., Jain, R. K., and Srirengalakshmi, M. 2022. Effect of nanoparticle coatings on frictional resistance of orthodontic archwires: a systematic review and meta-analysis. World J. Dent, 13 (4): 417–424.
[15] Nasim, I., Jabin, Z., Kumar, S. R., and Vishnupriya, V. 2022. Green synthesis of calcium hydroxide-coated silver nanoparticles using Andrographis paniculata and Ocimum sanctum Linn. leaf extracts: An antimicrobial and cytotoxic activity. J. Conserv. Dent. 25 (4): 369–374.
[16] Kamath, K. A., Nasim, I., and Rajeshkumar, S. 2020. Evaluation of the re-mineralization capacity of a gold nanoparticle-based dental varnish: An in vitro study. J. Conserv. Dent. 23 (4): 390–394.
[17] Maheswari, T. N. U., and Dhanvanth, M. 2022. Topical herbal therapeutic formulation used in the management of oral potentially malignant disorders – A systematic review. J. Indian Acad. Oral Med. Radiol. 34 (2): 223.
[18] Ashraf, K., Sultan, S., and Adam, A. 2018. Benth. is an Outstanding Food Medicine: Review of Phytochemical and Pharmacological Activities. J. Pharm. Bioallied Sci. 10 (3): 109–118.
[19] Raghuvanshi, D., Dhalaria, R., Sharma, A., Kumar, D., Kumar, H., Valis, M., … Puri, S. 2021. Ethnomedicinal Plants Traditionally Used for the Treatment of Jaundice (Icterus) in Himachal Pradesh in Western Himalaya-A Review. Plants, 10 (2): 232.
[20] Nisak, K., and Rini, C. S. 2021. Effectiveness of The Antibacterial Activity on Orthosiphon aristatus Leaves Extract Against Proteus mirabilis and Staphylococcus saprophyticus. Medicra (Journal of Medical Laboratory Science/Technology). 4 (2): 72-77.
[21] Ashraf, K., Sultan, S., and Adam, A. 2018. Orthosiphon stamineus Benth. is an outstanding food medicine: Review of phytochemical and pharmacological activities. J.Pharm.d Bioallied Sci.10 (3): 109-118
[22] Rajeshkumar, S., Lakshmi, T., and Tharani, M. 2021. Green synthesis of copper nanoparticles synthesized using black tea and its antibacterial activity against oral pathogens. Int. J. Dent. Oral Sci., 8 (9): 4156–4159.
[23] Rajeshkumar, S., and Lakshmi, S. (2021). Anticariogenic activity of silver nanoparticles synthesized using fresh leaves extract of kalanchoe pinnata. Int J Dentistry Oral Sci., 8 (7): 2985–2987.
[24] Nasim, I., Rajesh Kumar, S., Vishnupriya, V., and Jabin, Z. 2020. Cytotoxicity and anti-microbial analysis of silver and graphene oxide bio nanoparticles. Bioinformation, 16 (11): 831–836.
[25] Nasim, I., Rajeshkumar, S., and Vishnupriya, V. 2021. Green synthesis of reduced graphene oxide nanoparticles, its characterization, and antimicrobial properties against common oral pathogens. Int J Dentistry Oral Sci., 8 (2): 1670–1675.
[26] Sneka, and Santhakumar, P. 2021. Antibacterial Activity of Selenium Nanoparticles extracted from
Capparis decidua against Escherichia coli and Lactobacillus Species. J. Adv. Pharm. Technol. Res. 14 (8): 4452–4454.[27] Rieshy, V., Priya, J., Arivarasu, L., and Kumar, S. R. 2020. Enhanced Antimicrobial Activity Of Herbal Formulation Mediated Copper Nanoparticles Against Clinical Pathogens. Plant Cell Biotechnol. Mol. Biol. 21 (53-54): 52-56.
[28] Kishore, S. O. G., Priya, A. J., and Narayanan, L. 2020. Controlling of oral pathogens using turmeric and tulsi herbal formulation mediated copper nanoparticles. Plant Cell Biotechnol. Mol. Biol. 21 (53-54): 33-37
[29] Hussain, I., Singh, N. B., Singh, A., Singh, H., and Singh, S. C. 2015. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 38 (4): 545–560.
[30] Shinde, H. M., Bhosale, T. T., Gavade, N. L., Babar, S. B., Kamble, R. J., Shirke, B. S., and Garadkar, K. M. 2018. Biosynthesis of ZrO2 nanoparticles from Ficus benghalensis leaf extract for photocatalytic activity. J Mater. Sci. Mater. Electron. 29 (16): 14055–14064.
[31] Shanthi, S., and Sri Nisha Tharani, S. 2016. Green Synthesis of Zirconium Dioxide (ZrO2) Nanoparticles Using Acalypha Indica Leaf Extract. Int. J. Eng. Appl. Res. 3(4): 257689.
[32] Tabassum, N., Kumar, D., Verma, D., Bohara, R. A., and Singh, M. P. 2021. Zirconium oxide (ZrO2) nanoparticles from antibacterial activity to cytotoxicity: A next-generation of multifunctional nanoparticles. Mater. Today Comm. 26: 102156
[33] Al-Nema, L., and Al-Ali, A. 2022. Antifungal Activity of Magnesium Oxide and Zirconium Oxide Nanoparticles Incorporated into Alginate Impression Material. In Vitro Study. Al-Rafidain Dent. J. https://doi.org/10.33899/rdenj.2022.129812.1095
[34] Matteucci, C., Grelli, S., De Smaele, E., Fontana, C., and Mastino, A. 1999. Identification of nuclei from apoptotic, necrotic, and viable lymphoid cells by using multiparameter flow cytometry. Cytometry, 35 (2): 145–153.
Viewed PDF 2968 40 -
 Curcumin Coated Orthosiphon Stamineus Leaf Extract Based Selenium Nanoparticle for Potential Tissue Engineering ApplicationsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art005
Curcumin Coated Orthosiphon Stamineus Leaf Extract Based Selenium Nanoparticle for Potential Tissue Engineering ApplicationsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art005Curcumin Coated Orthosiphon Stamineus Leaf Extract Based Selenium Nanoparticle for Potential Tissue Engineering Applications
Abstract:
Curcumin is a type of polyphenol phytochemical that is bright yellow in colour and produced by the plant Curcuma longa. Despite its pharmacological properties, curcumin has low bioavailability, poor solubility, and undergoes rapid degradation. Nanoparticles (NPs) are used as a nanocarrier for drug delivery, to improve the stability and pharmacokinetics of the drug. Therefore, by coating curcumin over selenium NPs (SeNPs), the bioactivity, bioavailability, stability, and may increase the solubility of curcumin of SeNPs. This study aimed to synthesize the SeNPs from Orthosiphon stamineus leaf extract and coat it with curcumin and to characterize it and check its biocompatibility. Biosynthesis of SeNPs was carried out using plant extract of Evolvulus alsinoides and characterized using UV spectrophotometer, FT-IR, and SEM. Annexin V PI apoptotic and Hemolytic assay were used for checking biocompatibility. The UV-Vis spectrum gave a strong peak at 265 and 423 nm at various time intervals, indicating the SeNPs formation. Similarly, FT-IR has strong absorption bands at 3279, 1284, 1072, 1028, and cm−1 with wavelengths ranging from 4000-500 cm-1. SEM analysis of biosynthesized SeNPs showed a spherical shape. Our results suggest that curcumin-coated SeNPs possess greater biocompatibility towards PBMCs which was evaluated by Annexin V - PI assay and erythrocytes by hemolytic studies. Curcumin-coated Selenium nanoparticles were successfully synthesized by the biological method using leaf extract of Orthosiphon stamineus and reported as biocompatible using Flow cytometry. But a more detailed study should be done for implementing it in tissue engineering.
Keywords: Selenium nanoparticle, Curcumin, Orthosiphon stamineus, Tissue Engineering.Curcumin Coated Orthosiphon Stamineus Leaf Extract Based Selenium Nanoparticle for Potential Tissue Engineering Applications
References:
[1] Nabi, F., Arain, M. A., Hassan, F., Umar, M., Rajput, N., Alagawany, M., … Liu, J. 2020. Nutraceutical role of selenium nanoparticles in poultry nutrition: a review. Worlds Poult. Sci. J. 76 (3): 459–471.
[2] Pandiyan, I., Sri, S. D., Indiran, M. A., Rathinavelu, P. K., Prabakar, J., and Rajeshkumar, S. (2022). Antioxidant, anti-inflammatory activity of Thymus vulgaris-mediated selenium nanoparticles: An in vitro study. J. Conserv. Dent. 25 (3): 241–245.
[3] Sushanthi, S., Srisakthi, D., MeignanaArumugham, I., Pradeepkumar, R., and Rajeshkumar, S. 2021. Vernonia Amygdalina Mediated Copper Nanoparticles and its Characterization and Antimicrobial Activity - An In Vitro Study. Int J Dent. Oral Sci., 8 (7): 3330–3334.
[4] Maiyo, F., and Singh, M. 2017. Selenium nanoparticles: potential in cancer gene and drug delivery. Nanomedicine , 12 (9): 1075–1089.
[5] Maliael, M. T., Jain, R. K., and Srirengalakshmi, M. 2022. Effect of nanoparticle coatings on frictional resistance of orthodontic archwires: a systematic review and meta-analysis. World J. Dent, 13(4), 417–424.
[6] Nasim, I., Jabin, Z., Kumar, S. R., and Vishnupriya, V. (2022). Green synthesis of calcium hydroxide-coated silver nanoparticles using Andrographis paniculata and Ocimum sanctum Linn. leaf extracts: An antimicrobial and cytotoxic activity. JJ. Conserv. Dent. 25 (4): 369–374.
[7] Kamath, K. A., Nasim, I., and Rajeshkumar, S. (2020). Evaluation of the re-mineralization capacity of a gold nanoparticle-based dental varnish: An in vitro study. J. Conserv. Dent. 23 (4): 390–394.
[8] Maheswari, T. N. U., and Dhanvanth, M. 2022. Topical herbal therapeutic formulation used in the management of oral potentially malignant disorders – A systematic review. J. Indian Acad. Oral Med. Radiol. 34 (2): 223.
[9] Ghaderi, R. S., Adibian, F., Sabouri, Z., Davoodi, J., Kazemi, M., Amel Jamehdar, S., … Daroudi, M. 2022. Green synthesis of selenium nanoparticle by Abelmoschus esculentus extract and assessment of its antibacterial activity. Mater. Tech. 37(10), 1289–1297.
[10] Deng, W., Xie, Q., Wang, H., Ma, Z., Wu, B., and Zhang, X. 2017. Selenium nanoparticles as versatile carriers for oral delivery of insulin: Insight into the synergic antidiabetic effect and mechanism. Nanomed.: Nanotechnol. Biol. Med. 13 (6): 1965–1974.
[11] Pyrzynska, K., and Sentkowska, A. 2022. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostructure Chem.12 (4): 467–480.
[12] Sentkowska, A., and Pyrzyńska, K. 2022. The Influence of Synthesis Conditions on the Antioxidant Activity of Selenium Nanoparticles. Molecules , 27 (8): 2486
[13] Shirmehenji, R., Javanshir, S., and Honarmand, M. 2021. A Green Approach to the Bio-based Synthesis of Selenium Nanoparticles from Mining Waste. J. Cluster Sci. 32 (5): 1311–1323.
[14] Kora, A. J., and Rastogi, L. 2016. Biomimetic synthesis of selenium nanoparticles by Pseudomonas aeruginosa ATCC 27853: An approach for conversion of selenite. J. Environ. Manage. 181: 231–236.
[15] Meenambigai, K., Kokila, R., Chandhirasekar, K., Thendralmanikandan, A., Kaliannan, D., Ibrahim, K. S., … Nareshkumar, A. 2022. Green Synthesis of Selenium Nanoparticles Mediated by Nilgirianthus ciliates Leaf Extracts for Antimicrobial Activity on Foodborne Pathogenic Microbes and Pesticidal Activity Against Aedes aegypti with Molecular Docking. Biol. Trace Elem. Res. 200 (6): 2948–2962.
[16] Hatami, R., Javadi, A., & Jafarizadeh-Malmiri, H. (2020). Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization. Green Processing and Synthesis, 9(1), 685–692.
[17] Johnson, J., Shanmugam, R., & Lakshmi, T. (2022). A review on plant-mediated selenium nanoparticles and its applications. J. Popul. Ther. Clin. Pharmacol. 28 (2): e29–e40.
[18] Mi, X.-J., Choi, H. S., Perumalsamy, H., Shanmugam, R., Thangavelu, L., Balusamy, S. R., and Kim, Y.-J. 2022. Biosynthesis and cytotoxic effect of silymarin-functionalized selenium nanoparticles induced autophagy mediated cellular apoptosis via downregulation of PI3K/Akt/mTOR pathway in gastric cancer. Phytomedicine, 99: 154014.
[19] Ezhilarasan, D., Lakshmi, T., and Mallineni, S. K. 2022. Nano-based targeted drug delivery for lung cancer: therapeutic avenues and challenges. Nanomedicine , 17 (24): 1855–1869.
[20] Safaei, M., Mozaffari, H. R., Moradpoor, H., Imani, M. M., Sharifi, R., and Golshah, A. 2022. Optimization of Green Synthesis of Selenium Nanoparticles and Evaluation of Their Antifungal Activity against Oral Candida albicans Infection. Adv. Mater. Sci. Eng. 2022: 1376998
[21] Liu, W., Li, X., Wong, Y.-S., Zheng, W., Zhang, Y., Cao, W., and Chen, T. 2012. Selenium nanoparticles as a carrier of 5-fluorouracil to achieve anticancer synergism. ACS nano, 6 (8): 6578–6591.
[22] Nasim, I., Rajesh Kumar, S., Vishnupriya, V., and Jabin, Z. 2020. Cytotoxicity and anti-microbial analysis of silver and graphene oxide bio nanoparticles. Bioinformation, 16 (11): 831–836.
[23] Nasim, I., Rajeshkumar, S., and Vishnupriya, V. 2021. Green synthesis of reduced graphene oxide nanoparticles, its characterization, and antimicrobial properties against common oral pathogens. Int J Dentistry Oral Sci., 8 (2): 1670–1675.
[24] Kishore, S. O. G., Priya, A. J., and Narayanan, L. 2020. Controlling of oral pathogens using turmeric and tulsi herbal formulation mediated copper nanoparticles. Plant Cell Biotechnol. Mol. Biol. 21 (53-54): 33-37
[25] Rieshy, V., Priya, J., Arivarasu, L., and Kumar, S. R. 2020. Enhanced Antimicrobial Activity of Herbal Formulation Mediated Copper Nanoparticles Against Clinical Pathogens. Plant Cell Biotechnol. Mol. Biol. 21 (53-54): 52-56.
[26] Sneka, and Santhakumar, P. 2021. Antibacterial Activity of Selenium Nanoparticles extracted from Capparis decidua against Escherichia coli and Lactobacillus Species. J. Adv. Pharm. Tech. Res. 14 (8): 4452–4454.
[27] Palombo, E. A. 2011. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evid. Based. Complement. Alternat. Med. 2011: 680354.
[28] Rajeshkumar, S., Lakshmi, T., and Tharani, M. (2021). Green synthesis of copper nanoparticles synthesized using black tea and its antibacterial activity against oral pathogens. Int. J. Dent. Oral Sci., 8 (9): 4156–4159.
[29] Rajeshkumar, S., and Lakshmi, S. 2021. Anticariogenic activity of silver nanoparticles synthesized using fresh leaves extract of kalanchoe pinnata. . Int J Dentistry Oral Sci., 8 (7): 2985–2987.
[30] Twaij, B. M., and Hasan, M. N. 2022. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J.Plant Biol. 13 (1): 4–14.
[31] Sharifi-Rad, J., Rayess, Y. E., Rizk, A. A., Sadaka, C., Zgheib, R., Zam, W., … Martins, N. 2020. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 11: 01021.
[32] Ashraf, K., Sultan, S., and Adam, A. 2018. Orthosiphon stamineus Benth. is an Outstanding Food Medicine: Review of Phytochemical and Pharmacological Activities. J. Pharm. Bioallied Sci. 10 (3): 109–118.
[33] Wang, Q., Wang, J., Li, N., Liu, J., Zhou, J., Zhuang, P., and Chen, H. 2022. A Systematic Review of Orthosiphon stamineus Benth. in the Treatment of Diabetes and Its Complications. Molecules. 27 (2): 444
[34] Hossain, M. A., Ismail, Z., Rahman, A., and Kang, S. C. 2008. Chemical composition and anti-fungal properties of the essential oils and crude extracts of Orthosiphon stamineus Benth. Ind. Crops Prod. 27 (3): 328–334.
[35] Hossain, M. A., and Mizanur Rahman, S. M. 2015. Isolation and characterisation of flavonoids from the leaves of medicinal plant Orthosiphon stamineus. Arab. J. Chem. 8 (2): 218–221.
[36] Ameer, O. Z., Salman, I. M., Asmawi, M. Z., Ibraheem, Z. O., and Yam, M. F. 2012. Orthosiphon stamineus: traditional uses, phytochemistry, pharmacology, and toxicology. J. Med. Food. 15 (8):, 678–690.
[37] Guo, M., Li, Y., Lin, Z., Zhao, M., Xiao, M., Wang, C., … Zhu, B. 2017. Surface decoration of selenium nanoparticles with curcumin induced HepG2 cell apoptosis through ROS mediated p53 and AKT signaling pathways. RSC Adv. 7 (83): 52456–52464.
[38] Francis, S., Joseph, S., Koshy, E. P., and Mathew, B. 2017. Green synthesis and characterization of gold and silver nanoparticles using Mussaenda glabrata leaf extract and their environmental applications to dye degradation. Environ. Sci. Pollut. Res. 24 (21): 17347–17357.
[39] Bulmus, V., Woodward, M., Lin, L., Murthy, N., Stayton, P., and Hoffman, A. 2003. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J. Control. Release. 93(2), 105–120.
[40] Qi, B., Wang, C., Ding, J., and Tao, W. (2019). Editorial: Applications of Nanobiotechnology in Pharmacology. Front. Pharmacol. 10: 1451.
[41] Cittrarasu, V., Kaliannan, D., Dharman, K., Maluventhen, V., Easwaran, M., Liu, W. C.,
Arumugam, M. 2021. Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci. Rep. 11 (1): 1032.[42] Gunti, L., Dass, R. S., and Kalagatur, N. K. 2019. Phytofabrication of Selenium Nanoparticles from Emblica officinalis Fruit Extract and Exploring Its Biopotential Applications: Antioxidant, Antimicrobial, and Biocompatibility. Front. Microbiol. 10: 931.
[43] Ramamurthy, C. H., Sampath, K. S., Arunkumar, P., Suresh Kumar, M., Sujatha, V., Premkumar, K., and Thirunavukkarasu, C. 2013. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 36 (8): 1131-1139.
Viewed PDF 999 13 -
 Green Synthesis of Gold Nanoparticles Using Eucalyptus and Piper Longum and Its Subsequent Antioxidant Activity EvaluationAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art006
Green Synthesis of Gold Nanoparticles Using Eucalyptus and Piper Longum and Its Subsequent Antioxidant Activity EvaluationAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art006Green Synthesis of Gold Nanoparticles Using Eucalyptus and Piper Longum and Its Subsequent Antioxidant Activity Evaluation
Abstract:
Gold continues to be one of the oldest dental restorative materials which has been used for dental repairs for more than 4000 years and remains an important metal in the dental sector. In a world where the importance of nanoparticles has been well established and its preparation has become much easier, it is important to analyse if these nanoparticles can be extracted from a plant based source as well. Along with its extraction, assessment of each property of the nanoparticle is essential. A few ingredients used in Ayurveda which can also be found being used in almost every household is pepper and eucalyptus and over the years, its importance has remained constant, if not showing an increase. The aim of this study was to extract gold nanoparticles using Eucalyptus and Piper longum and evaluate the antioxidant activity of the derived gold nanoparticles. Preparation of plant extract was done following which, extraction of gold nanoparticles was performed. Antioxidant properties of the gold nanoparticles were tested by a DPPH assay method and compared against the antioxidant gold standard, butylated hydroxytoluene (BHT). The percentage of absorbance was calculated and data was analyzed. The results demonstrated the presence of elemental gold. Both, plant extract derived AuNPs exhibited significant free-radical scavenging activity indicating that they possess antioxidant effects.
Keywords - Eucalyptus, antioxidant, Nanoparticles, Novel technique, Piper longum.Green Synthesis of Gold Nanoparticles Using Eucalyptus and Piper Longum and Its Subsequent Antioxidant Activity Evaluation
References:
[1] Chen PC, Mwakwari SC, Oyelere AK. Gold nanoparticles: from nanomedicine to nanosensing. Nanotechnology, science, and applications. 2008; 1:45.
[2] Gielen M, Tiekink ER, editors. Metallotherapeutic drugs and metal-based diagnostic agents: the use of metals in medicine. John Wiley & Sons; 2005 Sep 1.
[3] Kumar PS, Pastoriza-Santos I, Rodríguez-González B, De Abajo FJ, Liz-Marzán LM. High-yield synthesis and optical response of gold nanostars. Nanotechnology. 2007 Nov 29;19(1):015606.
[4] Edelman ER, Seifert P, Groothuis A, Morss A, Bornstein D, Rogers C. Gold-coated NIR stents in porcine coronary arteries. Circulation. 2001 Jan 23;103(3):429-34.
[5] Svedman C, Tillman C, Gustavsson CG, Möller H, Frennby B, Bruze M. Contact allergy to gold in patients with gold‐plated intracoronary stents. Contact Dermatitis. 2005 Apr;52(4):192-6.
[6] Thelen A, Bauknecht HC, Asbach P, Schrom T. Behavior of metal implants used in ENT surgery in 7 Tesla magnetic resonance imaging. European Archives of Oto-Rhino-Laryngology and Head & Neck. 2006 Oct;263(10):900-5.
[7] Demann ET, Stein PS, Haubenreich JE. Gold as an implant in medicine and dentistry. Journal of long-term effects of medical implants. 2005;15(6).
[8] Wersäll PJ, Blomgren H, Pisa P, Lax I, Kälkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta oncologica. 2006 Jan 1;45(4):493-7.
[9] Shaw CF. Gold-based therapeutic agents. Chemical reviews. 1999 Sep 8;99(9):2589-600.
[10] Gielen M, Tiekink ER, editors. Metallotherapeutic drugs and metal-based diagnostic agents: the use of metals in medicine. John Wiley & Sons; 2005 Sep 1.
[11] Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N, Hong J. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007 Feb 6;18(10):105104.
[12] Wright DC, German RM, Gallant RF. Materials Science: Copper and Silver Corrosion Activity in Crown and Bridge Alloys. Journal of Dental Research. 1981 Apr;60(4):809-14.
[13] Knosp H, Holliday RJ, Corti CW. Gold in dentistry: alloys, uses and performance. Gold bulletin. 2003 Sep;36(3):93-102.
[14] Rieshy, V., Priya, J., Arivarasu, L., Kumar, S. R., & Devi, G. (2020). Enhanced Antimicrobial Activity of Herbal Formulation Mediated Copper Nanoparticles against Clinical Pathogens. Plant cell biotechnology and molecular biology, 21(53-54), 52–56.
[15] Kishore, S. O. G., Priya, A. J., Narayanan, L., Kumar, S. R., & Devi, G. (2020). Controlling of oral pathogens using turmeric and tulsi herbal formulation mediated copper nanoparticles. Plant cell biotechnology and molecular biology, 21(53-54), 33–37.
[16] Sneka S, Preetha Santhakumar. Antibacterial Activity of Selenium Nanoparticles extracted from Capparis decidua against Escherichia coli and Lactobacillus Species. Research Journal of Pharmacy and Technology. 2021; 14(8):4452-4. doi: 10.52711/0974-360X.2021.00773
[17] Roshan, A., Jothipriya, A., Arivarasu, l., Kumar, R., & Devi, G. (2020). Antifungal Activity of Tulsi and Turmeric assisted Copper Nano Particles. Plant cell biotechnology and molecular biology, 21(27-28), 9–13.
[18] Iffat Nasim, S. Rajeshkumar, V Vishnupriya. Green Synthesis of Reduced Graphene Oxide Nanoparticles, Its Characterization and Antimicrobial Properties against Common Oral Pathogens. Int J Dentistry Oral Sci. 2021;8(2):1670-1675
[19] Nasim I, Kumar SR, Vishnupriya V, Jabin Z. Cytotoxicity and anti-microbial analysis of silver and graphene oxide bio nanoparticles. Bioinformation. 2020;16(11):831.
[20] Rajeshkumar S, Lakshmi T. Anticariogenic Activity Of Silver Nanoparticles Synthesized Using Fresh Leaves Extract Of Kalanchoe Pinnata. Int J Dentistry Oral Sci. 2021 Jul 2;8(7):2985-7.
[21] Rajeshkumar S, Jayapriya J, Lakshmi T. A Review on plant mediated selenium nanoparticles and its applications: Selenium nanoparticles. Journal of Population Therapeutics and Clinical Pharmacology. 2021;28(2).
[22] Kamath KA, Nasim I, Rajeshkumar S. Evaluation of the re-mineralization capacity of a gold nanoparticle-based dental varnish: An in vitro study. Journal of conservative dentistry: JCD. 2020 Jul;23(4):390.
[23] Maliael MT, Jain RK, Srirengalakshmi M. Effect of nanoparticle coatings on frictional resistance of orthodontic archwires: a systematic review and meta-analysis. World. 2022;13(4).
[24] Chokkattu JJ, Mary DJ, Shanmugam R, Neeharika S. Embryonic Toxicology Evaluation of Ginger-and Clove-mediated Titanium Oxide Nanoparticles-based Dental Varnish with Zebrafish. The Journal of Contemporary Dental Practice. 2023 Mar 17;23(11):1157-62.
[25] Niveda Rajeshwaran JR, Rajeshkumar S. Evaluation of Antioxidant and Anti-Inflammatory Activity of Grape Seed Oil Infused with Silver Nano-particles an In Vitro Study. Int J Dentistry Oral Sci. 2021 Jul 15;8(7):3318-22.
[26] S.Sushanthi, Srisakthi Doraikannan, Meignana Arumugham Indiran, Pradeepkumar Rathinavelu, Rajeshkumar S. Vernonia Amygdalina Mediated Copper Nanoparticles and its Characterization and Antimicrobial Activity - An In vitro Study. Int J Dentistry Oral Sci. 2021;8(7):3330-3334. doi: http://dx.doi.org/10.19070/2377-8075-2100067
[27] S Rajeshkumar, T Lakshmi. Green Synthesis of Gold Nanoparticles Using Kalanchoe Pinnata and Its Free Radical Scavenging Activity. Int J Dentistry Oral Sci. 2021;8(7): 2981-2984.doi: dx.doi.org/10.19070/2377-8075-21000606.
[28] S Rajeshkumar, T Lakshmi. Anticariogenic Activity of Silver Nanoparticles Synthesized Using Fresh Leaves Extract Of Kalanchoe Pinnata. Int J Dentistry Oral Sci. 2021;8(7): 2985-2987.doi: dx.doi.org/10.19070/2377-8075-21000607.
[29] Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug discovery today. 2003 Dec 15;8(24):1112-20.
[30] de la Escosura-Muñiz A, Maltez-da Costa M, Sánchez-Espinel C, Díaz-Freitas B, Fernández-Suarez J, González-Fernández Á, Merkoçi A. Gold nanoparticle-based electrochemical magnetoimmunosensor for rapid detection of anti-hepatitis B virus antibodies in human serum. Biosensors and Bioelectronics. 2010 Dec 15;26(4):1710-4.
[31] Kawasaki ES, Player A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine: Nanotechnology, Biology and Medicine. 2005 Jun 1;1(2):101-9.
[32] Lee JH, Koo YK, Cho HW, Cha HJ, Shin DU, Oh TG, Lee SJ. Cysteine-loaded pH-responsive liposome/gold nanoparticles as a time-temperature indicator with instantaneous color change. Innovative Food Science & Emerging Technologies. 2021 Oct 1;73:102794.
[33] Gu YJ, Cheng J, Lin CC, Lam YW, Cheng SH, Wong WT. Nuclear penetration of surface functionalized gold nanoparticles. Toxicology and applied Pharmacology. 2009 Jun 1;237(2):196-204.
[34] Kumar SA, Peter YA, Nadeau JL. Facile biosynthesis, separation and conjugation of gold nanoparticles to doxorubicin. Nanotechnology. 2008 Nov 18;19(49):495101.
[35] Baron R, Šljukić B, Salter C, Crossley A, Compton RG. Electrochemical detection of arsenic on a gold nanoparticle array. Russian Journal of Physical Chemistry A. 2007 Sep;81(9):1443-7.
[36] Lalaoui N, Rousselot-Pailley P, Robert V, Mekmouche Y, Villalonga R, Holzinger M, Cosnier S, Tron T, Le Goff A. Direct electron transfer between a site-specific pyrene-modified laccase and carbon nanotube/gold nanoparticle supramolecular assemblies for bioelectrocatalytic dioxygen reduction. Acs Catalysis. 2016 Mar 4;6(3):1894-900.
[37] Chen PC, Mwakwari SC, Oyelere AK. Gold nanoparticles: from nanomedicine to nanosensing. Nanotechnology, science, and applications. 2008;1:45.
[38] Kumar G, Sahoo D. Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold. Journal of applied phycology. 2011 Apr;23(2):251-5.
[39] Lim Soo P, Sidorov SN, Mui J, Bronstein LM, Vali H, Eisenberg A, Maysinger D. Gold-labeled block copolymer micelles reveal gold aggregates at multiple subcellular sites. Langmuir. 2007 Apr 24;23(9):4830-6.
[40] Chen PC, Mwakwari SC, Oyelere AK. Gold nanoparticles: from nanomedicine to nanosensing. Nanotechnology, science, and applications. 2008;1:45.
[41] Ishida O, Maruyama K, Sasaki K, Iwatsuru M. Size-dependent extravasation, and interstitial localization of polyethyleneglycol liposomes in solid tumor-bearing mice. International journal of pharmaceutics. 1999 Nov 10;190(1):49-56.
[42] Zharov VP, Galitovskaya EN, Johnson C, Kelly T. Synergistic enhancement of selective nanophotothermolysis with gold nanoclusters: potential for cancer therapy. Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery. 2005 Sep;37(3):219-26.
[43] Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature physical science. 1973 Jan;241(105):20-2.
[44] Brown KR, Natan MJ. Hydroxylamine seeding of colloidal Au nanoparticles in solution and on surfaces. Langmuir. 1998 Feb 17;14(4):726-8.
[45] Gusta LV, O’connor BJ, Gao YP, Jana S. A re-evaluation of controlled freeze-tests and controlled environment hardening conditions to estimate the winter survival potential of hardy winter wheats. Canadian journal of plant Science. 2001 Apr 1;81(2):241-6.
[46] Busbee BD, Obare SO, Murphy CJ. An improved synthesis of high‐aspect‐ratio gold nanorods. Advanced Materials. 2003 Mar 4;15(5):414-6.
[47] Sau TK, Murphy CJ. Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir. 2004 Jul 20;20(15):6414-20.
[48] Sun W, Arculus RJ, Kamenetsky VS, Binns RA. Release of gold-bearing fluids in convergent margin magmas prompted by magnetite crystallization. Nature. 2004 Oct;431(7011):975-8.
[49] Kundu S, Panigrahi S, Praharaj S, Basu S, Ghosh SK, Pal A, Pal T. Anisotropic growth of gold clusters to gold nanocubes under UV irradiation. Nanotechnology. 2007 Jan 12;18(7):075712.
[50] Mitra RN, Das PK. In situ preparation of gold nanoparticles of varying shape in molecular hydrogel of peptide amphiphiles. The Journal of Physical
Chemistry C. 2008 Jun 5;112(22):8159-66.[51] Pyrpassopoulos S, Niarchos D, Nounesis G, Boukos N, Zafiropoulou I, Tzitzios V. Synthesis, and self-organization of Au nanoparticles. Nanotechnology. 2007 Nov 1;18(48):485604.
Viewed PDF 917 17 -
 Green Synthesis of Gold Nanoparticles Using Eucalyptus and Piper Longum and Its Subsequent Antiinflammatory Activity EvaluationAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art007
Green Synthesis of Gold Nanoparticles Using Eucalyptus and Piper Longum and Its Subsequent Antiinflammatory Activity EvaluationAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art007Green Synthesis of Gold Nanoparticles Using Eucalyptus and Piper Longum and Its Subsequent Antiinflammatory Activity Evaluation
Abstract:
Gold is the oldest dental restorative material, used for dental repairs for more than 4000 years and remains an important metal included in the dental sector. In a world where nanoparticle importance has been well established and preparation of nanoparticles has become much easier, it is important to assess if these nanoparticles can be extracted from plants as well. Along with its extraction, analysis of each property of the nanoparticle is essential. Pepper and eucalyptus remain two of the most important ingredients used in ayurveda and can be easily found in every household. The aim of this study was to extract gold nanoparticles using Eucalyptus and Piper longum and evaluate the antibacterial activity of the derived gold nanoparticles. Preparation of plant extract was done following which, extraction of gold nanoparticles was performed. Antiinflammatory properties of the gold nanoparticles were tested by albumin denaturation method and compared against the anti-inflammatory gold standard, Diclofenac sodium. The protein denaturation levels were measured, and the data was compiled. From this study, it can be concluded that gold nanoparticles derived from pepper and eucalyptus can be used as a potential source of anti-inflammatories.
Keywords - Eucalyptus, antiinflammatory, Nanoparticles, novel technique, Gold, Piper longum.Green Synthesis of Gold Nanoparticles Using Eucalyptus and Piper Longum and Its Subsequent Antiinflammatory Activity Evaluation
References:
[1] Chen PC, Mwakwari SC, Oyelere AK. Gold nanoparticles: from nanomedicine to nanosensing. Nanotechnology, science, and applications. 2008; 1:45.
[2] Gielen M, Tiekink ER, editors. Metallotherapeutic drugs and metal-based diagnostic agents: the use of metals in medicine. John Wiley & Sons; 2005 Sep 1.
[3] Kumar PS, Pastoriza-Santos I, Rodríguez-González B, De Abajo FJ, Liz-Marzán LM. High-yield synthesis and optical response of gold nanostars. Nanotechnology. 2007 Nov 29;19(1):015606.
[4] Edelman ER, Seifert P, Groothuis A, Morss A, Bornstein D, Rogers C. Gold-coated NIR stents in porcine coronary arteries. Circulation. 2001 Jan 23;103(3):429-34.
[5] Svedman C, Tillman C, Gustavsson CG, Möller H, Frennby B, Bruze M. Contact allergy to gold in patients with gold‐plated intracoronary stents. Contact Dermatitis. 2005 Apr;52(4):192-6.
[6] Thelen A, Bauknecht HC, Asbach P, Schrom T. Behavior of metal implants used in ENT surgery in 7 Tesla magnetic resonance imaging. European Archives of Oto-Rhino-Laryngology and Head & Neck. 2006 Oct;263(10):900-5.
[7] Demann ET, Stein PS, Haubenreich JE. Gold as an implant in medicine and dentistry. Journal of long-term effects of medical implants. 2005;15(6).
[8] Wersäll PJ, Blomgren H, Pisa P, Lax I, Kälkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta oncologica. 2006 Jan 1;45(4):493-7.
[9] Shaw CF. Gold-based therapeutic agents. Chemical reviews. 1999 Sep 8;99(9):2589-600.
[10] Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N, Hong J. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007 Feb 6;18(10):105104.
[11] Wright DC, German RM, Gallant RF. Materials Science: Copper and Silver Corrosion Activity in Crown and Bridge Alloys. Journal of Dental Research. 1981 Apr;60(4):809-14.
[12] Knosp H, Holliday RJ, Corti CW. Gold in dentistry alloys, uses and performance. Gold bulletin. 2003 Sep;36(3):93-102
[13] Rieshy, V., Priya, J., Arivarasu, L., Kumar, S. R., & Devi, G. (2020). Enhanced antimicrobial activity of herbal formulation mediated copper nanoparticles against clinical pathogens. Plant cell biotechnology and molecular biology, 21(53-54), 52–56.
[14] Kishore, S. O. G., Priya, A. J., Narayanan, L., Kumar, S. R., & Devi, G. (2020). Controlling of oral pathogens using turmeric and tulsi herbal formulation mediated copper nanoparticles. Plant cell biotechnology and molecular biology, 21(53-54), 33–37.
[15] Sneka S, Preetha Santhakumar. Antibacterial Activity of Selenium Nanoparticles extracted from Capparis decidua against Escherichia coli and Lactobacillus Species. Research Journal of Pharmacy and Technology. 2021; 14(8):4452-4. doi: 10.52711/0974-360X.2021.00773
[16] Roshan, A., Jothipriya, A., Arivarasu, L., Kumar, R., & Devi, G. (2020). Antifungal activity of tulsi and turmeric assisted copper nano particles. Plant cell biotechnology and molecular biology, 21(27-28), 9–13.
[17] Iffat Nasim, S. Rajeshkumar, V Vishnupriya. Green Synthesis of Reduced Graphene Oxide Nanoparticles, Its Characterization and Antimicrobial Properties against Common Oral Pathogens. Int J Dentistry Oral Sci. 2021;8(2):1670-1675
[18] Nasim I, Kumar SR, Vishnupriya V, Jabin Z. Cytotoxicity and anti-microbial analysis of silver and graphene oxide bio nanoparticles. Bioinformation. 2020;16(11):831.
[19] Rajeshkumar S, Lakshmi T. Anticariogenic Activity Of Silver Nanoparticles Synthesized Using Fresh Leaves Extract Of Kalanchoe Pinnata. Int J Dentistry Oral Sci. 2021 Jul 2;8(7):2985-7.
[20] Rajeshkumar S, Jayapriya J, Lakshmi T. A Review on plant mediated selenium nanoparticles and its applications: Selenium nanoparticles. Journal of Population Therapeutics and Clinical Pharmacology. 2021;28(2).
[21] Kamath KA, Nasim I, Rajeshkumar S. Evaluation of the re-mineralization capacity of a gold nanoparticle-based dental varnish: An in vitro study. Journal of conservative dentistry: JCD. 2020 Jul;23(4):390.
[22] Maliael MT, Jain RK, Srirengalakshmi M. Effect of nanoparticle coatings on frictional resistance of orthodontic archwires: a systematic review and meta-analysis. World. 2022;13(4).
[23] Chokkattu JJ, Mary DJ, Shanmugam R, Neeharika S. Embryonic Toxicology Evaluation of Ginger-and Clove-mediated Titanium Oxide Nanoparticles-based Dental Varnish with Zebrafish. The Journal of Contemporary Dental Practice. 2023 Mar 17;23(11):1157-62.
[24] NivedaRajeshwaran JR, Rajeshkumar S. Evaluation of Antioxidant and Anti-Inflammatory Activity of Grape Seed Oil Infused with Silver Nano-particles an In Vitro Study. Int J Dentistry Oral Sci. 2021 Jul 15;8(7):3318-22.
[25] S.Sushanthi, Srisakthi Doraikannan, Meignana Arumugham Indiran, Pradeepkumar Rathinavelu, Rajeshkumar S. Vernonia Amygdalina Mediated Copper Nanoparticles and their Characterization and Antimicrobial Activity - An In vitro Study. Int J Dentistry Oral Sci. 2021;8(7):3330-3334. doi: http://dx.doi.org/10.19070/2377-8075-2100067.
[26] S Rajeshkumar, T Lakshmi. Green Synthesis of Gold Nanoparticles Using Kalanchoe Pinnata and Its Free Radical Scavenging Activity. Int J Dentistry Oral Sci. 2021;8(7):2981-2984. doi: dx.doi.org/10.19070/2377-8075-21000606.
[27] Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug discovery today. 2003 Dec 15;8(24):1112-20.
[28] de la Escosura-Muñiz A, Maltez-da Costa M, Sánchez-Espinel C, Díaz-Freitas B, Fernández-Suarez J, González-Fernández Á, Merkoçi A. Gold nanoparticle-based electrochemical magnetoimmunosensor for rapid detection of anti-hepatitis B virus antibodies in human serum. Biosensors and Bioelectronics. 2010 Dec 15;26(4):1710-4.
[29] Kawasaki ES, Player A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine: Nanotechnology, Biology and Medicine. 2005 Jun 1;1(2):101-9.
[30] Lee JH, Koo YK, Cho HW, Cha HJ, Shin DU, Oh TG, Lee SJ. Cysteine-loaded pH-responsive liposome/gold nanoparticles as a time-temperature indicator with instantaneous color change. Innovative Food Science & Emerging Technologies. 2021 Oct 1; 73:102794.
[31] Gu YJ, Cheng J, Lin CC, Lam YW, Cheng SH, Wong WT. Nuclear penetration of surface functionalized gold nanoparticles. Toxicology and applied Pharmacology. 2009 Jun 1;237(2):196-204.
[32] Kumar SA, Peter YA, Nadeau JL. Facile biosynthesis, separation, and conjugation of gold nanoparticles to doxorubicin. Nanotechnology. 2008 Nov 18;19(49):495101.
[33] Baron R, Šljukić B, Salter C, Crossley A, Compton RG. Electrochemical detection of arsenic on a gold nanoparticle array. Russian Journal of Physical Chemistry A. 2007 Sep;81(9):1443-7.
[34] Lalaoui N, Rousselot-Pailley P, Robert V, Mekmouche Y, Villalonga R, Holzinger M, Cosnier S, Tron T, Le Goff A. Direct electron transfer between a site-specific pyrene-modified laccase and carbon nanotube/gold nanoparticle supramolecular assemblies for bioelectrocatalytic dioxygen reduction. Acs Catalysis. 2016 Mar 4;6(3):1894-900.
[35] Kumar G, Sahoo D. Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold. Journal of applied phycology. 2011 Apr;23(2):251-5.
[36] Lim Soo P, Sidorov SN, Mui J, Bronstein LM, Vali H, Eisenberg A, Maysinger D. Gold-labeled block copolymer micelles reveal gold aggregates at multiple subcellular sites. Langmuir. 2007 Apr 24;23(9):4830-6.
[37] Ishida O, Maruyama K, Sasaki K, Iwatsuru M. Size-dependent extravasation, and interstitial localization of polyethyleneglycol liposomes in solid tumor-bearing mice. International journal of pharmaceutics. 1999 Nov 10;190(1):49-56.
[38] Zharov VP, Galitovskaya EN, Johnson C, Kelly T. Synergistic enhancement of selective nanophotothermolysis with gold nanoclusters: potential for cancer therapy. Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery. 2005 Sep;37(3):219-26.
[39] Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature physical science. 1973 Jan;241(105):20-2.
[40] Brown KR, Natan MJ. Hydroxylamine seeding of colloidal Au nanoparticles in solution and on surfaces. Langmuir. 1998 Feb 17;14(4):726-8.
[41] Gusta LV, O’connor BJ, Gao YP, Jana S. A re-evaluation of controlled freeze-tests and controlled environment hardening conditions to estimate the winter survival potential of hardy winter wheats. Canadian journal of plant Science. 2001 Apr 1;81(2):241-6.
[42] Busbee BD, Obare SO, Murphy CJ. An improved synthesis of high‐aspect‐ratio gold nanorods. Advanced Materials. 2003 Mar 4;15(5):414-6.
[43] Sau TK, Murphy CJ. Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir. 2004 Jul 20;20(15):6414-20.
[44] Sun W, Arculus RJ, Kamenetsky VS, Binns RA. Release of gold-bearing fluids in convergent margin magmas prompted by magnetite crystallization. Nature. 2004 Oct;431(7011):975-8.
[45] Kundu S, Panigrahi S, Praharaj S, Basu S, Ghosh SK, Pal A, Pal T. Anisotropic growth of gold clusters to gold nanocubes under UV irradiation. Nanotechnology. 2007 Jan 12;18(7):075712.
[46] Mitra RN, Das PK. In situ preparation of gold nanoparticles of varying shape in molecular hydrogel of peptide amphiphiles. The Journal of Physical Chemistry C. 2008 Jun 5;112(22):8159-66.
[47] Pyrpassopoulos S, Niarchos D, Nounesis G, Boukos N, Zafiropoulou I, Tzitzios V. Synthesis, and self-organization of Au nanoparticles. Nanotechnology. 2007 Nov 1;18(48):485604.
[48] P. Manonmani, M. Ramar, N. Geetha, M. Valan Arasu, R. Rasik Erusan, R. Mari Selvam, J. Jerlin Sowmiya. Synthesis of silver nanoparticles using natural products from Acalypha Indica (Kuppaimeni) and curcuma longa (Turmeric) on antimicrobial activities. M Ramar, IJPRBS, 2015; Volume 4(1): 151-164
[49] J.R. Vane, R.M. Botting, New insight into the mode of action of anti-inflammatory drugs, Inflamm. Res. 44 (1995) 1–10.
[50] E. Yesilada, O. Ustun, E. Sezik, Y. Takaishi, Y. Ono, G. Honda, Inhibitory effect of turkish folk remedies on inflammatory cytokines: Interleukins-1alpha, interleukins-1beta and tumour necrosis factor alpha, J. Ethnopharmacol. 58 (1997) 59–73.
[51] E.L. Opie, On the relation of necrosis and inflammation to denaturation of proteins, J. Exp. Med. 115 (1962) 597-608.
[52] E. Umapathy, E.J. Ndebia, A. Meeme, B. Adam, P. Menziwa, B.N. Nkeh-Chungag et al., An experimental evaluation of Albucasetosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation, J. Med. Plants Res. 4 (2010) 789-795.
[53] Mizushima Y. Screening test for antirheumatic drugs. Lancet. 1966; 288:443.
Viewed PDF 892 15 -
 Effect of Diosmin on The Expression of Epithelial-Mesenchymal Transition Signaling Molecules in Ndea-Induced Hepato-Cellular Carcinoma in Experimental RatsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art008
Effect of Diosmin on The Expression of Epithelial-Mesenchymal Transition Signaling Molecules in Ndea-Induced Hepato-Cellular Carcinoma in Experimental RatsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art008Effect of Diosmin on The Expression of Epithelial-Mesenchymal Transition Signaling Molecules in Ndea-Induced Hepato-Cellular Carcinoma in Experimental Rats
Abstract:
Hepatocellular carcinoma (HCC) is a primary liver cancer, distinct from other cancers originating in other organs. Previous study demonstrated that diosmin exhibits anticancer effects by influencing the expression of apoptotic signaling molecules in NDEA-induced hepatocellular carcinoma in rats. However, its impact on the epithelial-mesenchymal transition (EMT) signaling pathway, crucial in liver cancer progression, remains unknown. The research aimed to investigate diosmin’s effects on EMT signaling molecule expression in NDEA-induced hepatocellular carcinoma in rats. In this experiment, adult male albino rats were categorized into three groups: a control group, NDEA-induced hepatocellular carcinogenic rats and rats with HCC treated with diosmin orally for 28 days. Liver function markers (AST and ALT) were done by biochemical analysis while mNRA expression analysis of EMT-signaling molecules ( E-cadherin and vimentin) were analyzed by Real Time-RT-PCR analysis. One-Way-ANOVA was used for the statistical analysis and significance was considered at p<0.05. Diosmin treatment resulted in a significant decrease in liver function markers compared to the control group (p<0.05). Moreover, diosmin administration led to a notable reduction in mRNA levels of EMT signaling molecules, specifically E-cadherin and vimentin, indicating its potential chemopreventive role against liver cancer. Findings of the present study concludes that diosmin, an alkaloid, may merge as a promising candidate for hepatocellular carcinoma treatment based on its demonstrated efficacy in this experimental model.Keywords: Novel method, Hepatocellular carcinoma, EMT signaling, diosmin, wistar rats, liver function, innovative technique.Effect of Diosmin on The Expression of Epithelial-Mesenchymal Transition Signaling Molecules in Ndea-Induced Hepato-Cellular Carcinoma in Experimental Rats
References:
[1] Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. https://doi.org/10.3322/caac.21492.
[2] Mittal, S., and El-Serag, H. B. 2013. Epidemiology of hepatocellular carcinoma: consider the population. Journal of clinical gastroenterology, 47 Suppl(0): S2–6.
[3] Hanin, S. M. A., Dharman, S., and Girija, A. S. S. 2022. Association of salivary microbes with oral mucositis among patients undergoing chemoradiotherapy in head and neck cancer: A hospital-based prospective …. Journal of International Oral Health. Retrieved from https://www.jioh.org/article.asp?issn=0976-7428;year=2022;volume=14;issue=1;spage=53;epage=60;aulast=Azima.
[4] Bhoori, S., and Mazzaferro, V. 2014. Corrigendum to “Current challenges in liver transplantation for hepatocellular carcinoma”. Best Practice & Research Clinical Gastroenterology, 28 (2014): 867–879. https://doi.org/10.1016/j.bpg.2014.10.007.
[5] Liberal, R., and Grant, C. R. 2016. Cirrhosis and autoimmune liver disease: Current understanding. World journal of hepatology, 8(28): 1157–1168.
[6] Blagotinsek, K., and Rozman, D. 2017. Targeting Signalling Pathways in Hepatocellular Carcinoma. Current pharmaceutical design, 23(1): 170–175.
[7] Abijeth, B., and Ezhilarasan, D. 2020. Syringic acid induces apoptosis in human oral squamous carcinoma cells through mitochondrial pathway. Journal of oral and maxillofacial pathology: JOMFP, 24(1): 40–45.
[8] Hu, T.H., Huang, C.C., Lin, P.R., Chang, H.W., Ger, L.P., Lin, Y.W.,Changchien, C.S., Lee, C.M., Tai, M.H. 2003. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer, 97(8): 1929–1940.
[9] Sushma, B., Vishnu Priya, V., Karthik Ganesh, M., and Gayathri, R. 2020. Awareness on risk factors associated with oral cancer among school students in chennai. International journal of current research and review, 12(24): S–87–S94.
[10] Sun, E. J., Wankell, M., Palamuthusingam, P., McFarlane, C., and Hebbard, L. 2021. Targeting the PI3K/Akt/mTOR Pathway in Hepatocellular Carcinoma. Biomedicines. https://doi.org/10.3390/biomedicines9111639.
[11] Sivakumar, N., Geetha, R. V., and Priya, V. 2021. Gayathri R, Dhanraj Ganapathy. Targeted Phytotherapy for Reactive Oxygen Species Linked Oral Cancer. Int J Dentistry Oral Sci, 8(1): 1425–1429.
[12] Knowledge about the effects of medicinal plants against COVID-19 among dental students-A questionnaire study. (n.d.). Retrieved from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-995148.
[13] Karthik, E. V. G., Priya, V. V., Gayathri, R., Dhanraj, G. 2021. Health Benefits Of Annona Muricata-A Review. Int J Dentistry Oral Sci, 8(7): 2965–2967.
[14] Lakshmi, T. (n.d.). Medicinal value and oral health aspects of acacia catechu-an update. International journal of dentistry and oral science.
[15] Lewinska, A., Siwak, J., Rzeszutek, I., and Wnuk, M. 2015. Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicology in vitro: an international journal published in association with BIBRA, 29(3): 417–425.
[16] Perumal, S., and Langeswaran, K. 2020. Diosmin anti-tumour efficacious against Hepatocellular Carcinoma. Research Journal of Pharmacy and Technology. https://doi.org/10.5958/0974-360x.2020.00308.x.
[17] Srinivasan, S., and Pari, L. 2012. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chemico-biological interactions, 195(1): 43–51.
[18] Queenthy, S. S., and John, B. 2013. Diosmin exhibits anti-hyperlipidemic effects in isoproterenol induced myocardial infarcted rats. European journal of pharmacology, 718(1-3): 213–218.
[19] Jayaraman, S., Krishnamoorthy, K., Prasad, M., Veeraraghavan, V.P., Krishnamoorthy, R., Alshuniaber, M.A., Gatasheh, M.K., Elrobh, M., and Gunassekaran. 2023. Glyphosate potentiates insulin resistance in skeletal muscle through the modulation of IRS-1/PI3K/Akt mediated mechanisms: An in vivo and in silico analysis. International Journal Biological Macromolecules, 242(Pt 2):124917.
[20] Selvaraj, J., Muthusamy, T., Srinivasan, C., and Balasubramanian, K. 2009. Impact of excess aldosterone on glucose homeostasis in adult male rat. Clinic Chimica Acta. 407(1-2):51-7.
[21] Lafaro, K. J., Demirjian, A. N., and Pawlik, T. M. 2015. Epidemiology of hepatocellular carcinoma. Surgical oncology clinics of North America, 24(1): 1–17.
[22] Darvesh, A. S., and Bishayee, A. 2013. Chemopreventive and Therapeutic Potential of Tea Polyphenols in Hepatocellular Cancer. Nutrition and Cancer. https://doi.org/10.1080/01635581.2013.767367.
[23] Santos, N. P., Colaço, A., da Costa, R. M. G., Oliveira, M. M., Peixoto, F., and Oliveira, P. A. 2014. N-diethylnitrosamine mouse hepatotoxicity: Time-related effects on histology and oxidative stress. Experimental and Toxicologic Pathology. https://doi.org/10.1016/j.etp.2014.07.002.
[24] Sakthisekaran, D. 2011. Resveratrol interferes with N-nitrosodiethylamine-induced hepatocellular carcinoma at early and advanced stages in male
Wistar rats. Molecular Medicine Reports. https://doi.org/10.3892/mmr.2011.555.[25] Bishayee, A., Politis, T., and Darvesh, A. S. 2010. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treatment Reviews. https://doi.org/10.1016/j.ctrv.2009.10.002.
[26] Silvestro, L., Tarcomnicu, I., Dulea, C., Attili, N. R. B., Ciuca, V., Peru, D., and Savu, S. R. 2013. Confirmation of diosmetin 3-O-glucuronide as major metabolite of diosmin in humans, using micro-liquid-chromatography–mass spectrometry and ion mobility mass spectrometry. Analytical and Bioanalytical Chemistry. https://doi.org/10.1007/s00216-013-7237-y.
[27] Ali, T. M., Abo-Salem, O. M., El Esawy, B. H., and El Askary, A. 2020. The Potential Protective Effects of Diosmin on Streptozotocin-Induced Diabetic Cardiomyopathy in Rats. The American Journal of the Medical Sciences. https://doi.org/10.1016/j.amjms.2019.10.005.
[28] Tanrikulu, Y., Şahin, M., Kismet, K., Kilicoglu, S. S., Devrim, E., Tanrikulu, C. S., Erdemli, E., Erel, S., Bayraktar, K., Akkus, M. A. 2013. The protective effect of diosmin on hepatic ischemia reperfusion injury: an experimental study. Bosnian Journal of Basic Medical Sciences. https://doi.org/10.17305/bjbms.2013.2305.
[29] Tahir, M., Rehman, M. U., Lateef, A., Khan, R., Khan, A. Q., Qamar, W, Ali, F., O’Hamiza, O., Sultana, S. 2013. Diosmin protects against ethanol-induced hepatic injury via alleviation of inflammation and regulation of TNF-α and NF-κB activation. Alcohol. https://doi.org/10.1016/j.alcohol.2012.12.010.
[30] Carlson, B. A., Dubay, M. M., Sausville, E. A., Brizuela, L., and Worland, P. J. 1996. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer research, 56(13): 2973–2978.
Viewed PDF 872 15 -
 Fabrication, Characterization, Antibacterial and Biocompatibility Studies of Graphene Oxide Loaded Alginate Chitosan Scaffolds for Potential Biomedical ApplicationsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art009
Fabrication, Characterization, Antibacterial and Biocompatibility Studies of Graphene Oxide Loaded Alginate Chitosan Scaffolds for Potential Biomedical ApplicationsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art009Fabrication, Characterization, Antibacterial and Biocompatibility Studies of Graphene Oxide Loaded Alginate Chitosan Scaffolds for Potential Biomedical Applications
Abstract:
Graphene oxide nanomaterial possesses greater biocompatibility. Chitosan alginate is produced from chitin by deacetylation, it is a biodegradable and biocompatible biomaterial. Graphene has a large specific surface area that enhances the antibacterial effect by enabling biocompatible interactions with bacterial membranes. To fabricate a biocompatible graphene oxide loaded alginate chitosan scaffold for potential biomedical uses and perform the characterization, antibacterial, and biocompatibility properties of ALG-CHI-GO scaffolds. The scaffolds were prepared by mixing the solution of ChitosanHCl (5 %) and graphene oxide-oxidized alginate (10 %). This gelled mixture is freeze-dried (lyophilization) to form the scaffold and is later characterized using FTIR and SEM, The scaffolds were then tested for in biocompatibility towards peripheral blood mononuclear cells and antibacterial properties against Enterococcus faecalis and Streptococcus mutans. The biocompatibility towards peripheral blood mononuclear was checked using the annexin V PI assay. To conclude that the fabricated Graphene oxide loaded chitosan alginate scaffold was found to be biocompatible and showed antibacterial properties.
Keywords: Antibacterial activity, Biocompatibility, Chitosan alginate scaffolds, Graphene oxide.Fabrication, Characterization, Antibacterial and Biocompatibility Studies of Graphene Oxide Loaded Alginate Chitosan Scaffolds for Potential Biomedical Applications
References:
[1] Yu, J. R., Navarro, J., Coburn, J. C., Mahadik, B., Molnar, J., Holmes, J. H., and Fisher, J. P. 2019. Current and Future Perspectives on Skin Tissue Engineering: Key Features of Biomedical Research, Translational Assessment, and Clinical Application. Advanced healthcare materials, 8(5): e1801471.
[2] John, S., Kesting, M. R., Paulitschke, P., Stöckelhuber, M., and von Bomhard, A. 2019. Development of a tissue-engineered skin substitute on a base of human amniotic membrane. Journal of tissue engineering, 10: 2041731418825378.
[3] Shi, Q., Luo, X., Huang, Z., Midgley, A. C., Wang, B., Liu, R., and Wang, K. 2019. Cobalt-mediated multi-functional dressings promote bacteria-infected wound healing. Acta biomaterialia, 86: 465–479.
[4] Ren, X., Han, Y., Wang, J., Jiang, Y., Yi, Z., Xu, H., and Ke, Q. 2018. An aligned porous electrospun fibrous membrane with controlled drug delivery - An efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta biomaterialia, 70: 140–153.
[5] Giuri, D., Barbalinardo, M., Sotgiu, G., Zamboni, R., Nocchetti, M., Donnadio, A., and Aluigi, A. 2019. Nano-hybrid electrospun non-woven mats made of wool keratin and hydrotalcites as potential bio-active wound dressings. Nanoscale, 11(13): 6422–6430.
[6] Gao, S., Tang, G., Hua, D., Xiong, R., Han, J., Jiang, S., and Huang, C. 2019. Stimuli-responsive bio-based polymeric systems and their applications. Journal of materials chemistry. B, Materials for biology and medicine, 7(5): 709–729.
[7] Ding, Q., Xu, X., Yue, Y., Mei, C., Huang, C., Jiang, S., and Han, J. 2018. Nanocellulose-Mediated Electroconductive Self-Healing Hydrogels with High Strength, Plasticity, Viscoelasticity, Stretchability, and Biocompatibility toward Multifunctional Applications. ACS applied materials & interfaces, 10(33): 27987–28002.
[8] Monica, K., Rajeshkumar, S., Ramasubramanian, A., Ramani, P., and Sukumaran, G. 2022. Anti-inflammatory and antimicrobial effects of herbal formulation using karpooravalli, mint, and cinnamon on wound pathogens. Journal of advanced pharmaceutical technology & research, 13(Suppl 2): S369–S373.
[9] Duraisamy, R., Ganapathy, D., and Shanmugam, R. 2021. Applications of chitosan in dental implantology - A literature review. International journal of dentistry and oral science, 8(9): 4140–4146.
[10] Tan, Q., Tang, H., Hu, J., Hu, Y., Zhou, X., Tao, Y., and Wu, Z. 2011. Controlled release of chitosan/heparin nanoparticle-delivered VEGF enhances regeneration of decellularized tissue-engineered scaffolds. International journal of nanomedicine, 6: 929–942.
[11] Rahmani Del Bakhshayesh, A., Annabi, N., Khalilov, R., Akbarzadeh, A., Samiei, M., Alizadeh, E., and Montaseri, A. 2018. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artificial cells, nanomedicine, and biotechnology, 46(4): 691–705.
[12] Rubio-Elizalde, I., Bernáldez-Sarabia, J., Moreno-Ulloa, A., Vilanova, C., Juárez, P., Licea-Navarro, A., & Castro-Ceseña, A. B. 2019. Scaffolds based on alginate-PEG methyl ether methacrylate-Moringa oleifera-Aloe vera for wound healing applications. Carbohydrate polymers, 206: 455–467.
[13] Mao, W., Kang, M. K., Shin, J. U., Son, Y. J., Kim, H. S., and Yoo, H. S. 2018. Coaxial Hydro-Nanofibrils for Self-Assembly of Cell Sheets Producing Skin Bilayers. ACS applied materials & interfaces, 10(50): 43503–43511.
[14] Chung, T. W., Yang, J., Akaike, T., Cho, K. Y., Nah, J. W., Kim, S. I., and Cho, C. S. (2002). Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials, 23(14): 2827–2834.
[15] Duraisamy, R., Ganapathy, D., and Shanmugam, R. 2021. Nanocomposites Used In Prosthodontics And Implantology - A Review. International journal of dentistry and oral science, 8(9): 4380–4387.
[16] Ng, I. M. J., and Shamsi, S. 2022. Graphene Oxide (GO): A Promising Nanomaterial against Infectious Diseases Caused by Multidrug-Resistant Bacteria. International journal of molecular sciences, 23(16): 9096.
[17] Balakrishnan, B., Soman, D., Payanam, U., Laurent, A., Labarre, D., and Jayakrishnan, A. 2017. A novel injectable tissue adhesive based on oxidized dextran and chitosan. (2017). Acta biomaterialia, 53: 343–354.
[18] Ravichandran, V., and Jayakrishnan, A. 2018. Synthesis and evaluation of anti-fungal activities of sodium alginate-amphotericin B conjugates. International journal of biological macromolecules, 108: 1101–1109.
[19] Aiswarriya, G. R., Gayathri, R., Veeraraghavan, V. P., Sankaran, K., and Francis, A. P. 2023. Green synthesis, characterization and biocompatibility study of quercetin-functionalized biogenic silver nanoparticles. Nano, 18(7): 2350055.
[20] Paxton, N. C., and Woodruff, M. A. 2022. Measuring contact angles on hydrophilic porous scaffolds by implementing a novel raised platform approach: A technical note. Polymers for advanced technologies, 33(10): 3759–3765.
[21] Ganesh, P. S., and Rai, V. R. 2015. Evaluation of Anti-bacterial and Anti-quorum Sensing Potential of Essential Oils Extracted by Supercritical CO2 Method Against Pseudomonas aeruginosa. Journal of Essential Oil Bearing Plants, 18(2): 264–275.
[22] Balaganesh, S., Kumar, P., Girija, A. S. S., and Rathinavelu, P. K. 2022. Probiotic curd as antibacterial agent against pathogens causing oral deformities - microbiological study. Journal of advanced pharmaceutical technology & research, 13(Suppl 2): S510–S513.
[23] Tamanna, I. S., Gayathri, R., Sankaran, K., Veeraraghavan, V. P., and Francis, A. P. (2023). Eco-friendly Synthesis of Selenium Nanoparticles Using Orthosiphon stamineus Leaf Extract and Its Biocompatibility Studies. BioNanoScience, 1–8 .https://doi.org/10.1007/s12668-023-01277-w
[24] Kolanthai, E., Sindu, P. A., Khajuria, D. K., Veerla, S. C., Kuppuswamy, D., Catalani, L. H., and Mahapatra, D. R. 2018. Graphene Oxide-A Tool for the Preparation of Chemically Crosslinking Free Alginate-Chitosan-Collagen Scaffolds for Bone Tissue Engineering. ACS applied materials & interfaces, 10(15): 12441–12452.
[25] Nowroozi, N., Faraji, S., Nouralishahi, A., and Shahrousvand, M. 2021. Biological and structural properties of graphene oxide/curcumin nanocomposite incorporated chitosan as a scaffold for wound healing application. Life sciences, 264: 118640.
[26] G., Y. D., V., Prabhu, A., Anil, S., and Venkatesan, J. 2021. Preparation and characterization of dexamethasone-loaded sodium alginate-graphene oxide microspheres for bone tissue engineering. Journal of drug delivery science and technology, 64: 102624.
[27] Zhang, J., Li, J., Jia, G., Jiang, Y., Liu, Q., Yang, X., and Pan, S. 2017. Improving osteogenesis of PLGA/HA porous scaffolds based on dual delivery of BMP-2 and IGF-1viaa polydopamine coating. RSC advances, 7(89): 56732–56742.
[28] Venkatesan, J., Lee, J.-Y., Kang, D. S., Anil, S., Kim, S.-K., Shim, M. S., and Kim, D. G. 2017. Antimicrobial and anticancer activities of porous chitosan-alginate biosynthesized silver nanoparticles. International journal of biological macromolecules, 98: 515–525.
[29] Sneka, and Santhakumar, P. 2021. Antibacterial Activity of Selenium Nanoparticles extracted from Capparis decidua against Escherichia coli and Lactobacillus Species. Journal of advanced pharmaceutical technology & research, 14(8): 4452–4454.
[30] de Faria, A. F., Martinez, D. S. T., Meira, S. M. M., de Moraes, A. C. M., Brandelli, A., Filho, A. G. S., and Alves, O. L. 2014. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids and surfaces. B, Biointerfaces, 113: 115–124.
[31] Anandhi, P., Tharani, M., Rajeshkumar, S., and Lakshmi, T. (2022). Antibacterial activity of cinnamon and clove oil against wound pathogens. Journal of population therapeutics and clinical pharmacology, 28(2): e41–e46.
[32] Kiew, S. F., Kiew, L. V., Lee, H. B., Imae, T., and Chung, L. Y. (2016). Assessing biocompatibility of graphene oxide-based nanocarriers: A review. Journal of controlled release, 226, 217–228.
Viewed PDF 883 8 -
 Biogenic Selenium Nanoparticles Loaded Alginate-Gelatin Scaffolds for Potential Tissue Engineering ApplicationsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art010
Biogenic Selenium Nanoparticles Loaded Alginate-Gelatin Scaffolds for Potential Tissue Engineering ApplicationsAuthor: Vishnu Priya VeeraraghavanDOI: 10.21522/TIJPH.2013.SE.23.01.Art010Biogenic Selenium Nanoparticles Loaded Alginate-Gelatin Scaffolds for Potential Tissue Engineering Applications
Abstract:
Selenium nanoparticles (SeNPs) were reported for its anticancer and antimicrobial properties. Alginate and gelatin scaffolds can act as an important biomaterial, more specifically in bone tissue engineering. Green synthesis of SeNPs from Luffa cylindrica (LC) and loading of SeNPS with alginate-gelatin scaffold and to check its biocompatibility. The SeNPs were prepared via the green synthesis method and loaded into an alginate-gelatin scaffold. Characterization studies such as UV-Vis spectroscopy, FTIR, and SEM were carried out in LC-SeNPs and Se-NPs loaded scaffold. The hydrophilicity of the scaffolds was determined using water contact angle measurements. Annexin V PI assay was conducted to determine the biocompatible nature of prepared SeNPs-loaded alginate-gelatin scaffolds. The UV-VIS spectrum gave an intense peak at 266 and 384 nm, whereas the FTIR gave a strong peak at 3500-500 cm-1 fingerprint regions. SEM images showed flower-shaped LC-SeNPs and their distribution of SeNPs on the surface of alginate-gelatin scaffolds. water contact angle measurement was found to be 29.21°. Cell viability results showed 78.11% viable cells following treatment with alg-gel-Se scaffold, revealing its biocompatibility towards peripheral blood mononuclear cells. Overall, it could be concluded that the SeNP-loaded alg-gel scaffold is a promising candidate for tissue engineering, but further studies are required to confirm its potential role.
Keywords- Selenium nanoparticles, Alginate-gelatin scaffolds, tissue engineering, biocompatibility.Biogenic Selenium Nanoparticles Loaded Alginate-Gelatin Scaffolds for Potential Tissue Engineering Applications
References:
[1] Johnson, J., Shanmugam, R., and Lakshmi, T. 2022. A review on plant-mediated selenium nanoparticles and its applications. Journal of population therapeutics and clinical pharmacology, 28(2): e29–e40.
[2] Ingole, A. R., Thakare, S. R., Khati, N. T., Wankhade, A. V., and Burghate, D. K. 2010. Green synthesis of selenium nanoparticles under ambient condition. Chalcogenide Lett, 7(7): 485–489.
[3] Naaziya, M., Biju, T. S., Francis, A. P., Veeraraghavan, V. P., Gayathri, R., and Sankaran, K. (2023). Synthesis, Characterization and in-vitro Biological Studies of Curcumin decorated Biogenic Selenium Nanoparticles. Nano LIFE. https://doi.org/10.1142/s1793984423500137
[4] Zhao, L., Wang, H., and Du, X. 2021. The therapeutic use of quercetin in ophthalmology: recent applications. Biomedicine & Pharmacotherapy. https://doi.org/10.1016/j.biopha.2021.111371
[5] Khurana, A., Tekula, S., Saifi, M. A., Venkatesh, P., and Godugu, C. 2019. Therapeutic applications of selenium nanoparticles. Biomedicine & pharmacotherapy 111: 802–812.
[6] Jayavarsha, V., Rajeshkumar, S., Lakshmi, T., and Sulochana, G. 2022. Green synthesis of selenium nanoparticles study using clove and cumin and its anti- inflammatory activity. Journal of complementary medicine research, 13(5): 84.
[7] Skalickova, S., Milosavljevic, V., Cihalova, K., Horky, P., Richtera, L., and Adam, V. 2017. Selenium nanoparticles as a nutritional supplement. Nutrition , 33: 83–90.
[8] Sneka, and Santhakumar, P. 2021. Antibacterial Activity of Selenium Nanoparticles extracted from Capparis decidua against Escherichia coli and Lactobacillus Species. Journal of advanced pharmaceutical technology & research, 14(8): 4452–4454.
[9] Ndwandwe, B. K., Malinga, S. P., and Kayitesi, E. 2021. Advances in green synthesis of selenium nanoparticles and their application in food packaging International Journal of Food Science & Technology https://doi.org/10.1111/ijfs.14916
[10] Ali, S. J., Preetha, Jeevitha, Prathap, L., and Rajeshkumar. 2020. Antifungal Activity of Selenium Nanoparticles Extracted from Capparis decidua Fruit against Candida albicans. Journal of evolution of medical and dental sciences, 9(34): 2452–2455. https://doi.org/10.14260/jemds/2020/533
[11] Partap, S., Kumar, A., Sharma, N. K., and Jha, K. K. 2012. Luffa Cylindrica : An important medicinal plant. J. Nat. Prod. Plant Resour., 2(1):127-134
[12] Al-Snafi, A. E. 2019. Constituents and pharmacology of Luffa cylindrica-A review. IOSR Journal of Pharmacy. 9(9): 68-79
[13] Akinwumi, K. A., Eleyowo, O. O., and Oladipo, O.O. 2021. A review on the ethnobotanical uses, phytochemistry and pharmacology effects of Luffa cylindrica. Natural Drugs from Plants. https://doi.org/10.5772/intechopen.98405
[14] Aboh, M. I., Okhale, S. E., & K., I. (2012). Preliminary studies on Luffa cylindrica: Comparative phytochemical and antimicrobial screening of the fresh and dried aerial parts. African journal of microbiology research, 3088, 3091. https://doi.org/10.5897/AJMR11.301
[15] Balakrishnan, N., and Sharma, A. 2013. Preliminary phytochemical and pharmacological activities of Luffa cylindrica L. fruit. Asian journal of pharmaceutical and clinical research, 6(2): 113–116.
[16] Soni, M., Gayathri, R., Sankaran, K., Veeraraghavan, V. P., and Francis, A. P. 2023. Green synthesis of selenium nanoparticles using luffa cylindrica and its biocompatibility for potential biomedical applications. Nano. 18(6): 2350042
[17] Cuadros, T. R., Erices, A. A., and Aguilera, J. M. 2015. Porous matrix of calcium alginate/gelatin with enhanced properties as scaffold for cell culture. Journal of the mechanical behavior of biomedical materials, 46: 331–342.
[18] Wang, L., Zhang, H. J., Liu, X., Liu, Y., Zhu, X., Liu, X., and You, X. 2021. A Physically Cross-Linked Sodium Alginate–Gelatin Hydrogel with High Mechanical Strength. ACS Applied Polymer Materials, 3(6): 3197–3205.
[19] You, F., Wu, X., and Chen, X. 2017. 3D printing of porous alginate/gelatin hydrogel scaffolds and their mechanical property characterization. International Journal of Polymeric Materials and Polymeric Biomaterials, 66(6): 299–306.
[20] Serafin, A., Murphy, C., Rubio, M. C., and Collins, M. N. 2021. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Materials science & engineering. C, Materials for biological applications, 122: 111927.
[21] Afjoul, H., Shamloo, A., and Kamali, A. 2020. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: An in vitro, in vivo study. Materials science & engineering. C, Materials for biological applications, 113, 110957.
[22] Luo, Y., Li, Y., Qin, X., Wa, Q. 2018. 3D printing of concentrated alginate/gelatin scaffolds with homogeneous nano apatite coating for bone tissue engineering. Materials & design, 146: 12–19.
[23] Bose, S., Roy, M., and Bandyopadhyay, A. 2012. Recent advances in bone tissue engineering scaffolds. Trends in biotechnology, 30(10): 546–554.
[24] Gour, A., and Jain, N. K. 2019. Advances in green synthesis of nanoparticles. Artificial cells, nanomedicine, and biotechnology , 47(1): 844–851.
[25] Hussain, I., Singh, N. B., Singh, A., Singh, H., and Singh, S. C. 2016. Green synthesis of nanoparticles and its potential application. Biotechnology letters, 38(4), 545–560.
[26] Alagesan, V., and Venugopal, S. 2019. Green Synthesis of Selenium Nanoparticle Using Leaves Extract of Withania somnifera and Its Biological Applications and Photocatalytic Activities. BioNanoScience, 9(1): 105–116.
[27] Sowndarya, P., Ramkumar, G., and Shivakumar, M. S. 2017. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artificial cells, nanomedicine, and biotechnology , 45(8): 1490–1495.
[28] Fardsadegh, B., and Jafarizadeh-Malmiri, H. 2019. Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their in vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Processing and Synthesis, 8(1): 399–407.
[29] Sharma, A., Puri, V., Kumar, P., and Singh, I. 2020. Rifampicin-Loaded Alginate-Gelatin Fibers Incorporated within Transdermal Films as a Fiber-in-
Film System for Wound Healing Applications. Membranes, 11(1):7[30] Leena, R. S., Vairamani, M., and Selvamurugan, N. 2017. Alginate/Gelatin scaffolds incorporated with Silibinin-loaded Chitosan nanoparticles for bone formation in vitro. Colloids and surfaces. B, Biointerfaces, 158, 308–318.
Viewed PDF 935 13

