Cathepsin D as a Biomarker in Colon Cancer Patients
Abstract:
Colon cancer is
a relatively common life-threatening malignancy for both sexes. Cancer cells
are depicted by the upregulation of lysosomal proteases – cathepsin D. The study
aimed to determine human cathepsin D activity in the control group as well as
its role in metastasis and invasion of colon cancer. Enzymatic assay(manual)
was used to measure the activity of cathepsin D and catalase, other
parameters were measured using analytical kits provided by reputable companies. Carcinoembryonic antigen CEA has been determined as a
biomarker for colon cancer, patients were subdivided into two classes based on
carcinoembryonic antigen values: group 1, ≤5 ng/mL group 2 >5 ng/mL. The
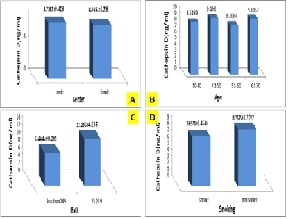
results explained that the normal value of Cathepsin D for the control group
was (8.61±0.294 ngml) and the activity of the enzyme was not affected by
gender, age, smoking
and BMI. The results also
proved a highly significant increase in cathepsin D activity in the patient's group
(17.81±0. 652 ng/ml) compared to the control. A remarkable elevation in the action
of cathepsin D in colon cancer patients was associated with a positive
relationship with carcinoembryonic antigen levels. This
supports the application of cathepsin D as a marker of tumor occurrence as well
as evidence of tumor metastasis and invasion after treatment in colon cancer
patients.
References:
[2].
Patel, S., Homaei, A., El-Seedi, H. R.,
Akhtar, N., 2018, Cathepsins: Proteases that are vital for survival but can
also be fatal. Biomedicine & Pharmacotherapy, 105, 526-532.
[3].
Rawlings, N. D., Barrett, A. J., Finn,
R., 2016, Twenty years of the MEROPS database of proteolytic enzymes, their
substrates and inhibitors. Nucleic acids research, 44(D1), D343-D350.
[4].
Metcalf, P., Fusek, M., 1993, Two
crystal structures for cathepsin D: the lysosomal targeting signal and active
site. The EMBO Journal, 12(4), 1293-1302.
[5].
Chen, S., Dong, H., Yang, S., Guo, H.,
2017, Cathepsins in digestive cancers. Oncotarget, 8(25), 41690.
[6].
Benes, P., Vetvicka, V., Fusek, M.,
2008, Cathepsin D—many functions of one aspartic protease. Critical reviews in
oncology/hematology, 68(1), 12-28.
[7].
Pranjol, M. Z. I., Gutowski, N. J.,
Hannemann, M., Whatmore, J. L., 2018, Cathepsin D non-proteolytically induces
proliferation and migration in human omental microvascular endothelial cells
via activation of the ERK1/2 and PI3K/AKT pathways. Biochimica et Biophysica
Acta (BBA)-Molecular Cell Research, 1865(1), 25-33.
[8].
Buck, M. R., Karustis, D. G., Day, N.
A., Honn, K. V., Sloane, B. F., 1992, Degradation of extracellular-matrix
proteins by human cathepsin B from normal and tumour tissues. Biochemical
Journal, 282(1), 273-278.
[9].
Yoshinari, M., Taurog, A., 1985,
Lysosomal digestion of thyroglobulin: role of cathepsin D and thiol proteases. Endocrinology,
117(4), 1621-1631.
[10]. Seo,
S. U., Woo, S. M., Im, S. S., Jang, Y., Han, E., Kim, S. H., Kwon, T. K., 2022,
Cathepsin D as a potential therapeutic target to enhance anticancer
drug-induced apoptosis via RNF183-mediated destabilization of Bcl-xL in cancer
cells. Cell Death & Disease, 13(2), 115.
[11]. Office
for National Statistics: Mortality Statistics-Deaths registered in England and
Wales Published online. 2014. [Available at: http://www.ons.gov.uk/ons/publications/allreleases.html?definition=tcm%3A77-27475
[12]. WHO.
Leading cause of death in Europe: fact sheet Copenhagen: WHO Regional Office
for Europe; 2012 [Available at: https://data.euro.who.int/hfadb]
[13]. Mohammadpour,
A. H., Salehinejad, Z., Elyasi, S., Mouhebati, M., Mirhafez, S. R., Samadi, S.,
Sahebkar, A., 2018, Evaluation of serum cathepsin D concentrations in coronary
artery disease. Indian Heart Journal, 70(4), 471-475.
[14]. Botteri,
E., Iodice, S., Bagnardi, V., Raimondi, S., Lowenfels, A. B., Maisonneuve, P.,
2008, Smoking and colorectal cancer: a meta-analysis. Jama, 300(23),
2765-2778.
[15]. Liang,
P. S., Chen, T. Y., Giovannucci, E., 2009, Cigarette smoking and colorectal
cancer incidence and mortality: Systematic review and meta‐analysis. International
Journal of cancer, 124(10), 2406-2415.
[16]. Meester,
R. G., Mannalithara, A., Lansdorp-Vogelaar, I., Ladabaum, U., 2019, Trends in
incidence and stage at diagnosis of colorectal cancer in adults aged 40 through
49 years, 1975-2015. Jama, 321(19), 1933-1934.
[17]. Burkitt,
D. P., 1971, Epidemiology of cancer of the colon and rectum. Cancer,
28(1), 3-13.
[18]. Giovannucci,
E., 2002, Modifiable risk factors for colon cancer. Gastroenterology
Clinics, 31(4), 925-943.
[19]. Hastuti,
S., 2024, Breast Cancer Screening Access Among Low-Income Women Under Social
Health Insurance: A Scoping Review. Public Health of Indonesia, 10(1), 21-32.
[20]. Thoke,
G. M., PP, A. S. U., Ganapathy, D., Sekar, D., 2024, Analysis of TGF-β Gene
Expression in Carboplatin Treated Lung Cancer Cells. exila International
Journal of Public Health, 12(3).
[21]. Kumar,
R. S., Amudha, P., Vidya, R., Kalpana, C. S., Sudhashini, S., 2024, A Review on
Anticancer Properties of Chebulagic Acid from Terminalia chebula. Texila
International Journal of Public Health, 12(3).
[22]. Eshrati
Yeganeh, F., Tabarzad, M., Khazraei, H., Bourbour, M., 2023, Synthesis and
evaluation of Escitalopram-loaded niosomes on colon cancer cell lines. Physiology
and Pharmacology, 27(3), 307-318.
[23]. Hariani,
H., Wiralis, W., Faturrahman, T. F. T., Suwarni, S., 2025, Medium Time Heating
of Syrop from Red Betel Leaf (Piper crocatum ruiz pav) Can Reduce
Carcinoembryonic Antigen (CEA) Level Among Adult Women in Southeast Sulawesi,
Indonesia. Public Health of Indonesia, 11(S1), 80-88.
[24]. Oliveira,
C. S. F., Pereira, H., Alves, S., Castro, L., Baltazar, F., Chaves, S. R.,
Côrte-Real, M., 2015, Cathepsin D protects colorectal cancer cells from
acetate-induced apoptosis through autophagy-independent degradation of damaged
mitochondria. Cell Death & Disease, 6(6), e1788-e1788.
[25]. Mijanovic,
O., Petushkova, A. I., Brankovic, A., Turk, B., Solovieva, A B., Nikitkina, A.
I., Zamyatnin Jr, A. A., 2021, Cathepsin D—managing the delicate balance. Pharmaceutics,
13(6), 837.
[26]. Skrzydlewska,
E., Sulkowska, M., Wincewicz, A., Koda, M., Sulkowski, S., 2005, Evaluation of
serum cathepsin B and D in relation to clinicopathological staging of
colorectal cancer. World Journal of Gastroenterology: WJG, 11(27), 4225.
[27]. Piecuch,
A., Kurek, J., Kucharzewski, M., Wyrobiec, G., Jasiński, D., Brzozowa-Zasada,
M., 2020, Catalase immunoexpression in colorectal lesions. Gastroenterology
Review/Przegląd Gastroenterologiczny, 15(4), 330-337.
[28]. Hong,
S. W., Lee, H. J., Han, K., Moon, J. M., Park, S., Soh, H., Kim, J. S., 2021,
Risk of gastrointestinal cancer in patients with an elevated level of
gamma-glutamyltransferase: A nationwide population-based study, PloS One,
16(2), e0245052.
[29]. Abdul‐Wahid,
A., Cydzik, M., Fischer, N. W., Prodeus, A., Shively, J. E., Martel, A.,
Gariépy, J., 2018, S erum‐derived carcinoembryonic antigen (CEA) activates
fibroblasts to induce a local re‐modeling of the extracellular matrix that
favors the engraftment of CEA‐expressing tumor cells. International Journal
of Cancer, 143(8), 1963-1977.
[30]. Digala1,
P., Muthu, S., Subramani, N., Duraisamy, N., Sundararaj, D., 2024, Understand
the Fatty Acid Metabolic Reprogramming of Immune Cells in Colorectal Cancer. Texila
International Journal of Public Health, 12(3): 1-8.

