Attenuation of Oxidative Stress and Anti-Alzheimer Effect of Ursolic Acid
Abstract:
Alzheimer’s disease (AD) is a progressive
neurodegenerative disorder characterized by cognitive decline and
neurodegeneration, with oxidative stress playing a pivotal role in its
pathophysiology. Ursolic acid (UA), a triterpenoid found in various medicinal
plants, exhibits antioxidant and neuroprotective properties that may counteract
oxidative damage associated with AD. This study aimed to evaluate the
antioxidant, neuroprotective, and anti-Alzheimer effects of Ursolic acid using
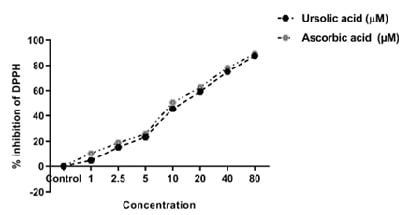
in vitro assays. The antioxidant potential was assessed via the DPPH free
radical scavenging assay. The neuroprotective effects were evaluated through
acetylcholinesterase inhibition assays, while UA's anti-Alzheimer potential was
examined using amyloid-beta aggregation and beta-secretase inhibition assays. Ursolic
acid demonstrated significant (p<0.001) antioxidant activity, effectively
scavenging DPPH radicals in a concentration-dependent manner. In the
acetylcholinesterase inhibition assay, UA exhibited a notable (p<0.05) reduction
in enzyme activity from 10 mM to the maximum concentration of 80mM, suggesting its potential to enhance cholinergic
neurotransmission. Furthermore, UA significantly inhibited amyloid-beta
aggregation and reduced beta-secretase activity between concentrations of 10 mM – 80 mM, indicating its promising role in mitigating key
pathological features of Alzheimer’s disease. The findings suggest that Ursolic
acid possesses potent antioxidant and neuroprotective effects, along with the
ability to inhibit amyloid-beta aggregation and beta-secretase activity. These
results highlight the therapeutic potential of Ursolic acid as a candidate for
the prevention and treatment of Alzheimer’s disease, warranting further
investigation in vivo models to validate its efficacy and mechanisms of action.
References:
[1]. Breijyeh, Z., & Karaman, R., 2020,
Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules,
25(24), 5789. doi: 10.3390/molecules25245789.
[2]. Serrano-Pozo, A.,
Frosch, M. P., Masliah, E., & Hyman, B. T., 2011, Neuropathological
alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine,
1(1), a006189. doi: 10.1101/cshperspect.a006189.
[3]. Spires-Jones, T. L.,
& Hyman, B. T., 2014, The intersection of amyloid beta and tau at synapses
in Alzheimer’s disease. Neuron, 82(4), 756-771. doi:
10.1016/j.neuron.2014.05.004.
[4]. Guo, T., Zhang, D., Zeng, Y., Huang, T.
Y., Xu, H., & Zhao, Y., 2020, Molecular and cellular mechanisms underlying
the pathogenesis of Alzheimer’s disease. Molecular neurodegeneration, 15, 1-37.
doi: 10.1186/s13024-020-00391-7.
[5]. Parameswari, R.P., Ramesh, G.,
Chidambaram, S. B., & Thangavelu, L., 2022, Oxidative stress mediated
neuroinflammation induced by chronic sleep restriction as triggers for
Alzheimer’s disease. Alzheimer's & Dementia, 18, e059016. DOI:10.1002/alz.059016
[6]. Singh, B., Day, C. M., Abdella, S.,
& Garg, S., 2024, Alzheimer's disease current therapies, novel drug
delivery systems and future directions for better disease management. Journal
of Controlled Release, 367, 402-424. doi: 10.1016/j.jconrel.2024.01.047.
[7]. Cetin, S., Knez, D., Gobec, S., Kos, J.,
& Pišlar, A., 2022, Cell models for Alzheimer’s and Parkinson’s disease: At
the interface of biology and drug discovery. Biomedicine &
Pharmacotherapy, 149, 112924. doi: 10.1016/j.biopha.2022.112924.
[8]. Jager, S., Trojan,
H., Kopp, T., Laszczyk, M. N., & Scheffler, A., 2009, Pentacyclic
triterpene distribution in various plants–rich sources for a new group of
multi-potent plant extracts. Molecules, 14(6), 2016-2031. https://doi.org/10.3390/molecules14062016
[9]. Kadasah, S. F., &
Radwan, M. O., 2023, Overview of ursolic acid potential for the treatment of
metabolic disorders, autoimmune diseases, and cancers via nuclear receptor
pathways. Biomedicines, 11(10), 2845. doi: 10.3390/biomedicines11102845.
[10]. Liu, J., 1995, Pharmacology of oleanolic
acid and ursolic acid. Journal of Ethnopharmacology, 49(2), 57-68. doi:
10.1016/0378-8741(95)90032-2.
[11]. Seo, D. Y., Lee, S. R., Heo, J. W., No,
M. H., Rhee, B. D., Ko, K. S., Kwak, H.B., Han, J., 2018, Ursolic acid in
health and disease. The Korean Journal of Physiology & Pharmacology: Official
Journal of the Korean Physiological Society and the Korean Society of
Pharmacology, 22(3), 235. doi: 10.4196/kjpp.2018.22.3.235.
[12]. Liang, W., Zhao, X.,
Feng, J., Song, F., & Pan, Y., 2016, Ursolic acid attenuates
beta-amyloid-induced memory impairment in mice. Arquivos de
neuro-psiquiatria, 74(6), 482-488. doi: 10.1590/0004-282X20160065.
[13]. Koleva, I. I., Van Beek, T. A., Linssen, J. P., Groot, A.
D., & Evstatieva, L. N., 2002, Screening of plant extracts for antioxidant
activity: a comparative study on three testing methods. Phytochemical
Analysis: An International Journal of Plant Chemical and Biochemical Techniques,
13(1), 8-17. doi: 10.1002/pca.611.
[14].
Ellman, G. L., Courtney, K.
D., Andres Jr, V., & Featherstone, R. M., 1961, A new and rapid
colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology,
7(2), 88-95. doi: 10.1016/0006-2952(61)90145-9.
[15]. Katayama, S., Sugiyama, H., Kushimoto, S.,
Uchiyama, Y., Hirano, M., & Nakamura, S., 2016, Effects of sesaminol
feeding on brain Aβ accumulation in a senescence-accelerated mouse-prone 8. Journal
of Agricultural and Food Chemistry, 64(24), 4908-4913. doi:
10.1021/acs.jafc.6b01237.
[16]. Miyazaki, H., Okamoto, Y.,
Motoi, A., Watanabe, T., Katayama, S., Kawahara, S. I., Makabe, H., Fujii, H.,
Yonekura, S., 2019, Adzuki bean (Vigna angularis) extract reduces amyloid-β
aggregation and delays cognitive impairment in Drosophila models of Alzheimer's
disease. Nutr. Res. Pract, 13, 64-69. doi: 10.4162/nrp.2019.13.1.64.
[17]. Panyatip, P., Tadtong, S.,
Sousa, E., & Puthongking, P., 2020, BACE1 inhibitor, neuroprotective, and
neuritogenic activities of melatonin derivatives. Scientia Pharmaceutica,
88(4), 58. doi.org/10.3390/scipharm88040058
[18]. Zhao, M., Wu, F.,
Tang, Z., Yang, X., Liu, Y., Wang, F., & Chen, B., 2023, Anti-inflammatory
and antioxidant activity of ursolic acid: A systematic review and
meta-analysis. Frontiers in Pharmacology, 14, 1256946.
doi.org/10.3389/fphar.2023.1256946
[19]. Pritam, P., Deka, R.,
Bhardwaj, A., Srivastava, R., Kumar, D., Jha, A. K., Jha, N. K., Villa, C.,
Jha, S. K., 2022, Antioxidants in Alzheimer’s disease: Current therapeutic
significance and future prospects. Biology, 11(2), 212. doi: 10.3390/biology11020212
[20]. Mirza, F. J., &
Zahid, S., 2022, Ursolic acid and rosmarinic acid ameliorate alterations in
hippocampal neurogenesis and social memory induced by amyloid beta in mouse
model of Alzheimer’s disease. Frontiers in Pharmacology, 13, 1058358.
doi.org/10.3389/fphar.2022.1058358.
[21]. Peitzika, S. C.,
& Pontiki, E., 2023, A review on recent approaches on molecular docking
studies of novel compounds targeting acetylcholinesterase in Alzheimer disease.
Molecules, 28(3), 1084. doi: 10.3390/molecules28031084.
[22]. García-Ayllón, M. S.,
Small, D. H., Avila, J., & Sáez-Valero, J., 2011, Revisiting the role of
acetylcholinesterase in Alzheimer’s disease: cross-talk with P-tau and
β-amyloid. Frontiers in molecular neuroscience, 4, 22.
doi.org/10.3389/fnmol.2011.00022.
[23]. Mlala, S., Oyedeji,
A. O., Gondwe, M., & Oyedeji, O. O., 2019, Ursolic acid and its derivatives
as bioactive agents. Molecules, 24(15), 2751. doi:
10.3390/molecules24152751.
[24]. Piccialli, I.,
Tedeschi, V., Caputo, L., D’Errico, S., Ciccone, R., De Feo, V., Secondo, A.,
Pannaccione, A., 2022, Exploring the therapeutic potential of phytochemicals in
Alzheimer’s disease: Focus on polyphenols and monoterpenes. Frontiers in
Pharmacology, 13, 876614.
[25]. Mugundhan, V.,
Arthanari, A., & Parthasarathy, P. R., 2024, Protective Effect of Ferulic
Acid on Acetylcholinesterase and Amyloid Beta Peptide Plaque Formation in
Alzheimer’s Disease: An In Vitro Study. Cureus, 16(2). doi:
10.7759/cureus.54103.
[26]. Das, B., & Yan,
R., 2019, A close look at BACE1 inhibitors for Alzheimer’s disease treatment. CNS
Drugs, 33(3), 251-263. doi: 10.1007/s40263-019-00613-7.

