Molecular Docking and ADME Profiling of 5-(Substituted Benzylidene)-2-(Arylamino)-1,3-Thiazol-4(5H)-ones: Insights into Pharmacokinetics and Binding Interactions
Abstract:
In the quest for effective cancer therapeutics, the
optimization of pharmacokinetics, toxicity profiles, and efficacy is crucial.
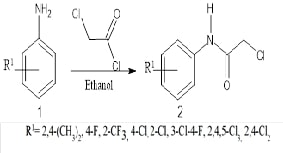
This study introduces a novel series of 5-(substituted
benzylidene)-2-(arylamino)-1,3-thiazol-4(5H)-ones, synthesized to explore their
potential as anti-cancer agents. These compounds were specifically designed
based on the promising anti-tumour activity of 5-arylidene-4-thiazolidinone
derivatives, known for their efficacy against MDA-MB-231 (human breast
cancer cell line). To assess these new
thiazol-4-ones, we used sophisticated in silico methods to perform
pharmacokinetic ADME predictions and molecular docking simulations. Our
molecular docking studies utilized FlexX to compare the binding affinities of
these compounds with known drugs: Gestrinone (targeting EGFR alpha for breast
cancer), Vandetanib (targeting VEGFR-2), and KU0058948 (targeting Poly ADP
ribose polymerase for ovarian cancer). These comparative analyses revealed
significant interactions with these key cancer targets. In addition, ADME
predictions were performed using the iLOG predictor from Swiss ADMET software,
demonstrating favourable properties for absorption, distribution, and
bioavailability. Interestingly, compounds with fluorine substitutions at
positions 2 or 4 of the acylamino ring showed encouraging activity and
satisfied Lipinski and Veber's rules-based drug-likeness requirements,
indicating that they could make good candidates for therapeutics. Furthermore,
these compounds showed low toxicity levels, enhancing their suitability for
further development.
References:
[1].
Alam, M. K., Alqhtani, N. R., Alnufaiy, B., et al., 2024, A systematic
review and meta-analysis of the impact of resveratrol on oral cancer: potential
therapeutic implications. BMC Oral Health, 24, 412.
[2]. Aboul-Fadl, T., Radwan, A.
A., Attia, M. I., Al-Dhfyan, A., Abdel-Aziz,
H.A. 2012, Schiff bases of indoline-2,3-dione
(isatin) with potential antiproliferative activity. Chem Cent J, 6, 49.
[3]. Pourbasheer, E., Amanlou, M., 2014, 3D-QSAR analysis of anti-cancer agents by CoMFA and CoMSIA.
Med Chem Res, 23, 800-809.
[4].
Seboletswe, P., Cele, N.,
Singh,
P., 2023, Thiazolidinone-heterocycle frameworks: A concise review of their pharmacological significance. ChemMedChem, 18, e202200618.
[5].
Lesyk,
R., Zimenkovsky, B., Atamanyuk, D., Jensen, F., Kiec-Kononowicz,
K.,
Gzell, A., 2006, Anticancer
thiopyrano[2,3-d] [1,3] thiazol-2-ones with norbornane moiety. Synthesis,
cytotoxicity, physico-chemical properties, and computational studies. Bioorg Med
Chem, 14, 5230-5240.
[6]. Kaminskyy,
D., Zimenkovsky, B., Lesyk, R., 2009, Synthesis
and in vitro anticancer activity of
2,4-azolidinedione-acetic acids derivatives.
Eur J Med Chem, 44, 3627-3636.
[7].
Vicini,
P., Geronikaki, A., Anastasia, K., Incerti,
M., Zani, F., 2006, Synthesis and
antimicrobial activity of novel 2-thiazolylimino-5-arylidene-4-thiazolidinones.
Bioorg Med Chem, 14, 3859-3864.
[8].
Elbarbary,
A. A., Khodair, A. L., Pedersen, E.
B., Nielsen, C, 1994, Synthesis and evaluation of
antiviral activity of 2′-deoxyuridines with 5-methylene-2-thiohydantoin
substituents in the 5-position. Monatsh Chem,
125, 593-598.
[9].
Rydzik,
E., Szadowska, A., Kaminska,
A., 1984, Synthesis of benzylidene derivatives
of 3-o, 3-m and 3-p-chlorophenylhydantoin and the study of their anticonvulsant
action.
Acta Pol Pharm, 41, 459-464.
[10]. Liu, H. L., Li, Z. C., Anthonsen, T. 2000, Synthesis and Fungicidal Activity of
2-Imino-3-(4-arylthiazol-2-yl)-thiazolidin-4-ones and Their 5-Arylidene
Derivatives. Molecules, 5, 1055-1061.
[11]. Samir, B., Wesam,
K., Ahmed, A. F., 2007, Synthesis and antimicrobial evaluation of some new thiazole,
thiazolidinone and thiazoline derivatives starting from 1-chloro-3,
4-dihydronaphthalene-2-carboxaldehyde. Eur J Med Chem, 42, 948-954.
[12]. Zhou, H. Y., Wu,
S. H., Zhai, S. M., 2008, Design, Synthesis, cytoselective toxicity, structure–activity relationships, and pharmacophore of thiazolidinone derivatives targeting drug-resistantlLung cancer cells. J Med Chem, 51, 1242-1251.
[13]. Muegge, I., Rarey,
M., 2001, Small molecule docking and scoring. Rev Computation Chem, 17, 1.
[14]. Irie, T., Sawa, M., Ito, S.,
Tanaka, C., Ro, S. G., Park, C. H., 2010. PCT. Int Appl, 2010122979.
[15]. Kumar, H.,
Aggarwal, N., Marwaha, M. G., et al. 2022. Thiazolidin-2,4-dione scaffold: An insight into recent advances as antimicrobial, antioxidant, and hypoglycemic agents. Molecules, 27, 6763.
[16]. Chawla, P., Singh,
R., Saraf, S. K.,
2012, Effect of
chloro and fluoro groups on the antimicrobial activity of 2,5-disubstituted
4-thiazolidinones: a comparative study. Med
Chem Res, 21, 3263-3271.
[17]. Thompson, M. A., 2004, Molecular docking using arguslab,
an efficient shape-based search algorithm and the scoring function.
ACS meeting, Philadelphia.
[18]. Rarey, M., Kramer,
B., Lengauer, T., Klebe, G.
A., 1996, A fast flexible docking
method using an incremental construction algorithm, J. Mol. Biol. 261 (1996) 470-489.
[19]. Cheng, T.
M., Blundell, T.
L., Fernandez-Recio, J., 2008, Structural assembly
of two-domain proteins
by rigid-body docking, BMC Bioinform. 9 (2008) 441.
[20]. Totrov, M., Abagyan, R., 2008, Flexible ligand docking to multiple receptor
conformations: a practical
alternative. Curr Opin
Struct Biol,
18, 178-184.
[21]. Becke, A. D.,
1993, Density‐functional thermochemistry. III. The role of exact
exchange. J Chem Phys, 98, 5648-5652.
[22]. Daina, A., Michielin,
O., Zoete, V., 2014, iLOGP: a simple, robust, and efficient description of
n-octanol/water partition coefficient for drug design using the GB/SA approach.
J Chem Inf Model, 54, 3284-3301.
[23]. E.M. Krovat, T. Steindl,
T. Langer, Recent advances in docking and scoring. Current Computer-Aided Drug Design
10 (2005) 93-102.
[24]. J. Azizian, M.K. Mohammadi, O. Firuzi, N. Razzaghi-asl, R.
Miri, Synthesis,
biological activity and docking study of some new isatin Schiff base
derivatives, Med. Chem.
Res. 2 (2012) 3730-3740.
[25]. Potts, R.O., Guy, R. H., 1992, Predicting Skin
Permeability. Pharm Res, 9, 663-669.
[26]. Thompson, P. E., Manallack, D. T., Blaney,
F. E., Gallagher, T., 1992,
Conformational studies
on (+)-anatoxin-a and derivative. J Comput Aided Mol Des, 6, 287-298.
[27]. Madsen, U., Larsen,
K., Liljefors, P., 2016, Tommy, Textbook of drug design and discovery. Washington DC:
Taylor & Fracis, 8, 45.
[28]. Jones, G., Willett, P., 1995, Docking small-molecule
ligands into active sites. Curr Opin Biotechnol, 6, 652-656.
[29]. Saini, R. S., Binduhayyim, R. I. H., Gurumurthy, V., et al., 2024, In
silico assessment of biocompatibility and toxicity: molecular docking and
dynamics simulation of PMMA-based dental materials for interim prosthetic
restorations, J. Mater. Sci: Mater. Med. 35 (2024)
35,
28.
[30]. Penning, T. D., Zhu, G. D., Gong, J., et al., 2010, Optimization of phenyl-substituted benzimidazole carboxamide
poly (ADP-ribose) polymerase inhibitors: identification of
(S)-2-(2-fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benzimidazole-4-carboxamide
(A-966492), a highly potent and efficacious inhibitor. J Med Chem, 53, 3142-3153.

