Correlation of Tyrosine Hydroxylase, Orexin, and NFL Concentration with Parkinson's Disease
Abstract:
The research aimed to identify serum biochemical
variables that correlated to Parkinson's disease by the collection of 120 blood
samples from healthy patients males coming to Ibn Sina Teaching Hospital in
Mosul, from December to June 2023-2024, these samples were divided into several
groups, Parkinson group, donated symbol P that included (30) samples divided
into two subgroups according to age, P1 (50-65)years and P2 (66-80) years 15
blood sample for each, while Parkinson's family group donated symbol F,
included (30) samples aged between (25-50) years, in addition to the Control
group C that included (60) samples of Healthy people which divided into three subgroups,
C1 (50-65) years, C2 (66-80) years, and C3 subgroup (25-50) years. These groups
' biochemical serum markers tyrosine hydroxylase, orexin, and neurofilament
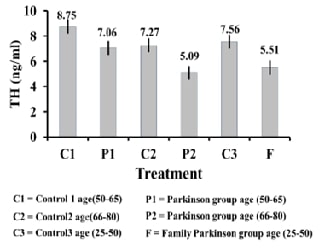
protein NFL had been compared. Results of this research showed a significant correlation
as TH enzyme concentration decreased in patients' serum with Parkinson's
disease in both groups P1, and P2 compared to the healthy group C1 and C2
(p≤0.01), also F group showed a significant decrease in the concentration of
this enzyme compared to the healthy group C3, Besides there was a substantial
difference in orexin hormone concentration level at probability level (p≤0.01))
in all groups, as hormone concentration decreased in P1subgroup patients
compared to healthy C1 patients of the same age group, and this decrease
increased in Parkinson's disease P2 patients compared to healthy C2. There was
a significant increase in the serum's concentration of NFL protein in the P1
subgroup compared to the healthy C1 subgroup at a probability level (P≤0.01).
References:
[1].
Aarsland, D., Batzu, L., Halliday, G. M., Geurtsen, G. J.,
Ballard, C., Ray Chaudhuri, K., Weintraub, D., 2021, Parkinson
disease-associated cognitive impairment. Nature Reviews Disease Primers,
7(1), 1-21. doi:10.1038/s41572-021-00280-3.
[2].
Bloem, B. R., Okun, M. S., Klein, C., 2021, Parkinson's
disease. The Lancet, 397(10291), 2284-2303. doi:10.1016/S0140-6736(21)00218-X.
[3].
Ismael, S. S., Al-Shamaa, S. D., 2020, Mutation in
microtubule-associated protein tau MAPT coding gene and its correlation with
Alzheimer’s disease, International Journal of Research in
Pharmaceutical Sciences, 11(4), 5150-5157, doi:10.26452/ijrps.v11i4.3119.
[4].
Calabresi, P., Mechelli, A., Natale, G., Volpicelli-Daley,
L., Di Lazzaro, G., Ghiglieri, V., 2023, Alpha-synuclein in Parkinson’s disease
and other synucleinopathies: from overt neurodegeneration back to early
synaptic dysfunction. Cell death & disease, 14(3), 176, doi:10.1038/s41419-023-05672-9.
[5].
Hamanaka, Y., Mizunami, M., 2019, Tyrosine
hydroxylase-immunoreactive neurons in the mushroom body of the field cricket,
Gryllus bimaculatus. Cell and tissue research, 376, 97-111, doi:10.1007/s00441-018-2969-9.
[6].
Sofy, S., Kakey, E., Alshamaa, S., 2014, The Protective Role of Green
Tae and Ginkgo biloba Extract against Aging Dysfunction Induced by D-Galactose
in Rats. Glob J Biol Agric Health Sci, 3(3), 97-101.
[7].
Borkar, C. D., Bharne, A. P., Nagalakshmi, B., Sakharkar, A. J.,
Subhedar, N. K., Kokare, D. M., 2018, Cocaine-and amphetamine-regulated
transcript peptide (CART) alleviates MK-801-induced schizophrenic dementia-like
symptoms. Neuroscience, 375, 94-107, doi:10.1016/j.neuroscience.2018.01.056.
[8].
Suzuki, K., Miyamoto, M., Miyamoto, T., Iwanami, M., Hirata,
K., 2011, Sleep Disturbances Associated with Parkinson′ s Disease. Parkinson’s
disease, 2011(1), 219056, doi:10.4061/2011/219056.
[9].
Justinussen, J. L., Egebjerg, C., Kornum, B. R., 2023, How
hypocretin agonists may improve the quality of wake in narcolepsy. Trends in
Molecular Medicine, 29(1), 61-69, doi:10.1016/j.molmed.2022.10.008.
[10]. Ng, A. S. L., Tan, Y. J.,
Yong, A. C. W., Saffari, S. E., Lu, Z., Ng, E. Y., Tan, E. K., 2020, Utility of
plasma Neurofilament light as a diagnostic and prognostic biomarker of the
postural instability gait disorder motor subtype in early Parkinson’s disease. Molecular
neurodegeneration, 15, 1-8, doi:10.1186/s13024-020-00385-5.
[11]. Halloway, S., Desai, P.,
Beck, T., Aggarwal, N., Agarwal, P., Evans, D., Chicago Health and Aging
Project, 2022, Association of neurofilament light with the development and severity
of Parkinson disease. Neurology, 98(22), e2185-e2193, doi:10.1212/WNL.0000000000200338.
[12]. Yi, L. X., Tan, E. K.,
& Zhou, Z. D., 2024, Tyrosine Hydroxylase Inhibitors and Dopamine Receptor
Agonists Combination Therapy for Parkinson’s Disease. International Journal
of Molecular Sciences, 25(9), 4643, doi:10.3390/ijms25094643.
[13]. Zhou, Z. D., Saw, W. T.,
Ho, P. G. H., Zhang, Z. W., Zeng, L., Chang, Y. Y., Tan, E. K., 2022, The role
of tyrosine hydroxylase–dopamine pathway in Parkinson’s disease pathogenesis. Cellular
and Molecular Life Sciences, 79(12), 599, doi:10.1007/s00018-022-04574-x.
[14]. Huang, S., Zhao, Z., Ma,
J., Hu, S., Li, L., Wang, Z., Zheng, J., 2021, Increased plasma orexin-A
concentrations are associated with the non-motor symptoms in Parkinson’s
disease patients. Neuroscience Letters, 741, 135480, doi:10.1016/j.neulet.2020.135480.
[15]. Braun, A., Manavis, J.,
Yamanaka, A., Ootsuka, Y., Blumbergs, P., Bobrovskaya, L., 2024, The role of
orexin in Parkinson's disease. Journal of Neuroscience Research, 102(3),
e25322, doi:10.1002/jnr.25322.
[16]. Oosterveld, L. P.,
Verberk, I. M., Majbour, N. K., El‐Agnaf, O. M., Weinstein, H. C., Berendse, H.
W., van de Berg, W.D., 2020, CSF or serum neurofilament light added to
α‐synuclein panel discriminates Parkinson's from controls. Movement
Disorders, 35(2), 288-295, doi:10.1002/mds.27897.
[17]. Pedersen, C. C., Ushakova,
A., Alves, G., Tysnes, O. B., Blennow, K., Zetterberg, H., Lange, J., 2024,
Serum neurofilament light at diagnosis: a prognostic indicator for accelerated
disease progression in Parkinson’s Disease. npj Parkinson's Disease, 10(1),
162, doi:10.1038/s41531-024-00768-1.
[18]. Nabizadeh, F.,
Mohamadzadeh, O., Hosseini, H., Rasouli, K., Afyouni, N. E., 2023, Serum
neurofilament light chain in LRRK2 related Parkinson’s disease: A five years
follow-up. Journal of Clinical Neuroscience, 110, 12-18, doi:10.1016/j.jocn.2023.01.015.
[19]. Teng, X., Mao, S., Wu,
H., Shao, Q., Zu, J., Zhang, W., Xu, C., 2023, The relationship between serum
neurofilament light chain and glial fibrillary acidic protein with the REM
sleep behavior disorder subtype of Parkinson's disease. Journal of
Neurochemistry, 165(2), 268-276, doi:10.1111/jnc.15780.
[20]. Praba, M. A.,
Venkataramaniah, K. G., Rashid, N. A., el Hasnaoul, R., Saadani, M. A. W.,
2024, Association of Cell Viability in Huntington Chorea Rat Models and the
Neuroprotective Role of Withania Somnifera in Public Health. Texila
International Journal of Public Health, 12(4), 1-10, doi:
10.21522/TIJPH.2013.12.04.Art076.

