Neuroprotective Efficacy of Eugenol Against Lead Acetate and Monosodium Glutamate Induced Neurotoxicity by Modulating Brain-Derived Neurotrophic Factor (BDNF) Gene Expression in Wistar Rats
Abstract:
The human nervous system is highly susceptible to various
environmental toxins, which can lead to neurodegenerative conditions
characterized by cognitive deficits, motor dysfunction, and even cell death.
Among these toxins, lead (Pb) and Monosodium Glutamate (MSG) have been evaluated
in this study for their neurotoxic effects. Lead exposure has been associated
with detrimental effects on the central nervous system, similarly, MSG, a
common food additive, has been reported to induce neurotoxicity through oxidative
stress and excitotoxicity mechanisms. Eugenol, found in essential oils, have
demonstrated promising antioxidant, anti-inflammatory and neuroprotective
properties. Hence Eugenol was used as a therapeutic agent against lead acetate
and MSG induced neurotoxicity by modulating Brain-derived Neurotrophic Factor. This
in vivo study involved 48 Wistar albino rats, divided into eight groups
consisting of Control, Lead acetate induction (100 mg/kg b.wt for 30 days), MSG
induction (2 g/kg b.wt for 21 days) and subsequent treatment with Eugenol (250
mg/kg b.wt for 30 days) in comparison with positive control, memantine (20mg/kg
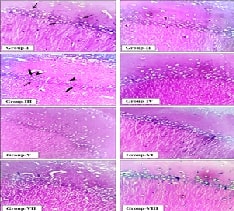
b.wt for 15 days). Histopathological and BDNF gene expression were evaluated
after the experimental period. Histopathological analysis confirmed that
eugenol preserved neuronal integrity, reducing neuronal damage caused by lead
acetate and MSG exposure by modulating free radical generation upon oxidative
stress. Eugenol treatment in rats exposed to lead and MSG resulted in a
significant upregulation of BDNF expression (p<0.01) compared to the
untreated toxin-exposed groups. These
outcomes suggest that Eugenol could be a possible therapeutic agent for
protecting the neuronal tissues from Lead acetate and MSG-induced
neurotoxicity.
References:
[1].
Flora,
G., Gupta, D., & Tiwari, A., 2012, Toxicity of lead: A review with recent
updates. Interdisciplinary Toxicology,
5(2), 47-58. https://doi.org/10.2478/v10102-012-0009-2
[2].
Ganesan,
B., Budin, S. B., & Anuar, I., 2022, Monosodium glutamate-induced oxidative
stress and cognitive impairments in rats: Neuroprotective effects of natural
antioxidants. Food and Chemical
Toxicology, 167, 113232. https://doi.org/10.1016%2FaCtox.2024.100148
[3].
Park,
S. E., Sapkota, K., Choi, J. H., 2011, Eugenol protects neuronal cells from
oxidative stress-induced apoptosis through TRPV1 activation. https://doi.org/10.3892%2Fetm.2020.9539
[4].
Meenakshi,
S., Varghese, S. S., Mohanraj K. G., 2023, Bone Regenerative Potential of a
Recombinant Parathormone Derivative in Experimentally Induced Critical-size
Calvarial Defects in Wistar Albino Rats. World J Dent 14(5):452–461.
[5].
Solmaz
Mohammad Inejad, 2017, “Pharmacological and toxicological properties of
eugenol” Turk J Pharm Sci, https://doi.org/10.4274%2Ftjps.62207
[6].
Prakash Binu, 2018, “Protective Effects of
Eugenol Against Hepatotoxicity Induced By Arsenic Trioxide: An Antileukemic
Drug” IJMS vol 43, no 3.
[7].
Hanna, S. S., Gazwi, 2020, “Mitigation of
lead neurotoxicity by the ethanolic extract of Laurus leaf in rats” Exotoxicol
Environ Saf Apr 1:192:110297. doi: 10.1016/j.ecoenv.2020.110297.
[8].
Fasakin, O. W., A. O. Fajobi, and O. O.
Oyedapo, 2017, "Neuroprotective potential of Aframomum melegueta extracts
on brain of monosodium glutamate-treated wistar albino rats." Journal of Neuroscience and Behavioral
Health 9.2 (2017): 16-27. DOI: 10.5897/JNBH2017.0145
[9].
Varsha Singh, 2013, “In vivo
antioxidative and neuroprotective effect of 4-Allyl-2- methoxyphenol against
chlorpyrifos-induced neurotoxicity in rat brain” Mol cell biochem,
Mar;388(1-2):61-74. doi:
10.1007/s11010-013-1899-9. Epub 2013 Dec 1.
[10]. Rajagopal shanmuga Sundaram, 2013,
“Neuroprotective potential of Ocimum sanctum (Linn) leaf extract in monosodium
glutamate induced excitotoxicity” African Journal of Pharmacy and
Pharmacology 7(27):1894-1906 DOI:10.5897/AJPP12.1445
[11]. Bancroft,
J. D., and Gamble, M., 2002, “Theory and Practice of Histological Techniques,”
Churchill Livingstone, London.
[12]. Sasi,
M., Vignoli, B., Canossa, M., & Blum, R., 2017, Neurobiology of local and
intercellular BDNF signaling. Pflügers
Archiv-European Journal of Physiology, 469(5), 593-610. https://doi.org/10.1007/s00424-017-1964-4
[13]. Patapoutian,
A., & Reichardt, L. F., 2001, Trk receptors: mediators of neurotrophin
action. Current Opinion in Neurobiology,
11(3), 272-280. https://doi.org/10.1016/s0959-4388(00)00208-7
[14]. Mahmoud,
S., Gharagozloo, M., Simard, C., Gris, D., 2019, Microglia and
neuroinflammation: modulation by exercise. Journal
of Neuroinflammation, 16, 138. https://doi.org/10.3389%2Ffnins.2023.1125428.
[15]. Pandiar,
D., Ramani, P., Krishnan R. P., Y., Dinesh, Histopathological analysis of soft
tissue changes in gingival biopsied specimen from patients with underlying
corona virus disease associated mucormycosis (CAM), https://doi.org/10.4317/medoral.25050.
[16]. Souparnika.V.,
Karthik Ganesh Mohanraj, Vidya, S., Antioxidant activity of L-Theanine on
Cadmium Induced oxidative stress mediated neurodegeneration-An invivo analysis,
https://doi.org/10.47750/jptcp.2022.952.
[17]. Sanders,
T., Liu, Y., Buchner, V., & Tchounwou, P. B., 2009, Neurotoxic effects and
biomarkers of lead exposure: A review. Reviews
on Environmental Health, 24(1), 15-45. https://doi.org/10.1515/reveh.2009.24.1.15.
[18]. Sanjay
Varshan, M., Lavanya Prathap, Selvaraj Jayaraman, Preetha, S., 2022, Anti
Proliferative Effect of Endogenous Dopamine Replica in Human Lung Cancer Cells
(A549) Via Pi3k and Akt Signalling Molecules. https://doi.org/10.47750/pnr.2022.13.S03.215.
[19]. Lu,
B., Nagappan, G., & Lu, Y., 2013, BDNF and synaptic plasticity, cognitive
function, and dysfunction. Handbook of
Experimental Pharmacology, 220, 223-250. https://doi.org/10.1007/978-3-642-45106-5_9.
[20]. Rattiner,
L. M., Davis, M., French, C. T., & Ressler, K. J. 2004, Brain-derived
neurotrophic factor and tyrosine kinase receptor B involvement in
amygdala-dependent fear conditioning. Journal
of Neuroscience, 24(20), 4796-4806. https://psycnet.apa.org/doi/10.1523/JNEUROSCI.5654-03.2004
[21]. Toscano,
C. D., & Guilarte, T. R., 2005, Lead neurotoxicity: From exposure to
molecular effects. Brain Research Reviews,
49(3), 529-554 https://doi.org/10.1016/j.brainresrev.2005.02.004.
[22]. Nagababu,
E., Rifkind, J. M., Boindala, S., & Nakka, L., 2010, Assessment of
antioxidant activity of eugenol in vitro and in vivo. Free Radical Biology and Medicine, 49(1), 144-153. https://doi.org/10.1007/978-1-60327-029-8_10.
[23]. Giridharan,
V. V., Thandavarayan, R. A., Sato, S., Ko, K. M., Ma, M., & Suzuki, K.,
2011, Eugenol attenuates neuroinflammatory responses and cognitive dysfunction
in a transgenic mouse model of Alzheimer's disease. Journal of Biological Chemistry, 286(43), 37716-37727. http://dx.doi.org/10.3109/10715762.2011.571682
[24]. Lu,
B., Nagappan, G., & Lu, Y., 2013, BDNF and synaptic plasticity, cognitive
function, and dysfunction. Handbook of
Experimental Pharmacology, 220, 223-250. https://doi.org/10.1007/978-3-642-45106-5_9
[25]. Saleh,
D. O., Baraka, S. M., Jaleel, G. A. A., Hassan, A., Ahmed-Farid, O. A., 2024,
Eugenol alleviates acrylamide-induced rat testicular toxicity by modulating
AMPK/p-AKT/mTOR signaling pathway and blood-testis barrier remodeling. Sci Rep.
2024 Jan 22;14(1):1910. doi: 10.1038/s41598-024-52259-1. PMID: 38253778; PMCID:
PMC10803763.
[26]. Allen,
S. J., Dawbarn, D., & Wilcock, G. K., 2011, BDNF levels in Alzheimer's
disease: implications for neuroprotective strategies. Journal of Alzheimer’s Disease, 22(1), 43-56. https://doi.org/10.2174%2F157015911798376190
[27]. Marvanová,
M., Menager, J., Bezard, E., & Bockaert, J., 2001, Reduced brain-derived
neurotrophic factor expression in the frontal cortex in Alzheimer's disease. Neurobiology of Aging, 22(2), 267-272. https://doi.org/10.1016/s0169-328x(97)00125-3
[28]. Dwivedi,
Y., Rizavi, H. S., Conley, R. R., Roberts, R. C., Tamminga, C. A., &
Pandey, G. N., 2003, Altered gene expression of brain-derived neurotrophic
factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Archives of General Psychiatry, 60(8),
804-815. https://doi.org/10.1001/archpsyc.60.8.804
[29]. Martinez-Herrera,
A., Pozos-Guillen, A., Ruiz-Rodriguez, S., Garrocho-Rangel, A.,
Vertiz-Hernandez, A., Escobar-Garcia, D. M., 2016, Effect of
4-allyl-1-hydroxy-2-methoxybenzene (eugenol) on inflammatory and apoptosis
processes in dental pulp fibroblasts. Mediators of Inflammation, 2016
doi: 10.1155/2016/9371403.9371403
[30]. Harb,
A. A., Bustanji, Y. K., Almasri, I. M., Abdalla, S. S., Eugenol, reduces LDL
cholesterol and hepatic steatosis in hypercholesterolemic rats by modulating
TRPV1 receptor. Scientific Reports. 2019;9(1):p. 14003. doi:
10.1038/s41598-019-50352-4.
[31]. Miranda,
M., Morici, J. F., Zanoni, M. B., Bekinschtein, P., Brain-Derived Neurotrophic
Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain.
Front. Cell. Neurosci. 2019;13:1–25. doi: 10.3389/fncel.2019.00363
[32]. Mitre,
M., Mariga, A., Chao, M. V., Neurotrophin Signalling: Novel Insights into
Mechanisms and Pathophysiology. Clin. Sci. 2017;131:13–23. doi:
10.1042/CS20160044.
[33]. Nisar,
M. F., Khadim, M, Rafiq, M., Chen J., Yang Y., Wan C. C., Pharmacological
Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxid Med
Cell Longev. 2021 Aug 3;2021:2497354. doi: 10.1155/2021/2497354. PMID:
34394824; PMCID: PMC8357497.
[34]. Ebenezer
Leonoline, J., Gunapriya, R., Ranganathan, K., Vijayaraghavan, R., Ganesh
Karthik, M., 2021, Determine Cyp17a1 and Ki67 Expressions in Pcos Induced Rat
Model Treated with Sepia pharaonis Ink Extract Proves Effective. Indian
Journal of Animal Research. 55(10): 1206-1214. doi: 10.18805/IJAR.B-4204.

