Potential Role of Anticancer Compounds Derived from Phytomedicines in Modulating the Signaling Pathways for Cancer Progression - A Review
Abstract:
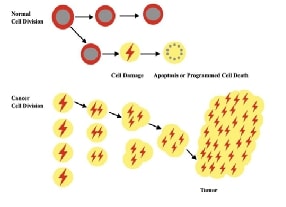
One of the most prevalent causes of death globally is cancer, which is a consequence of uncontrolled proliferation and division of cells in the body. The role of inflammation in tumour progression has been increasingly established. It also affects epigenetic changes that promote tumour induction and promotes all stages of carcinogenesis. Chronic inflammation may result in greater damage to DNA, interfere with DNA repair mechanisms, accelerate cellular division, and induce apoptosis, angiogenesis, and invasion of the tissue. A comprehensive knowledge of the cellular and molecular signaling mechanisms of tumor-endorsing inflammation is essential for the advancement of anti-cancer medications which concentrate on the interaction between malignancy formation and inflammatory mechanisms. Several inflammatory signalling pathways have been identified as regulating inflammation, including the NF-κB signalling pathway, the JAK-STAT signalling pathway, the MAPK signalling, the PI3K/AKT/mTOR signalling and the Wnt signalling cascade. Several phytochemicals can treat cancer by altering these pathways. There are numerous classes of phytochemicals in herbal medicine that are being used therapeutically. Herbal medicine has shown to be especially beneficial for cancer patients, with many reporting a considerable increase in survivorship as a result of treatment. Cellular metabolism, tumour development, growth, proliferation, metastasis, and cytoskeletal reorganization are all regulated by aberrations in different cellular signalling pathways. The primary emphasis of the current review focuses on the phytochemical’s capacity to combat cancer through modifying numerous cell signalling pathways.References:
[1]. “What Is Cancer? was originally published
by the National Cancer Institute” updated on October 11, 2021.
[2]. Madihalli Somashekharaiah Chandraprasad,
Abhijit Dey, Mallappa Kumara Swamy, 2022, 1 - Introduction to cancer and treatment
approaches, Editor(s): Mallappa Kumara Swamy, T. Pullaiah, Zhe-Sheng Chen, Paclitaxel, Academic Press, pp. 1-27, ISBN 9780323909518, https://doi.org/10.1016/B978-0-323-90951-8.00010-2
[3]. Jayaraman, S., Natarajan, S. R.,
Veeraraghavan, V. P., Jasmine, S., 2023, Unveiling
the anti-cancer mechanisms of calotropin: Insights into cell growth inhibition,
cell cycle arrest, and metabolic regulation in human oral squamous carcinoma
cells (HSC-3).
Journal of Oral Biology and Craniofacial Research. Nov 1;13(6):704-13.
[4]. Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., Bray, F., 2020, Global Cancer Statistics: GLOBOCAN Estimates
of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. May;71(3):209-249. doi: 10.3322/caac.21660.
Epub 2021 Feb 4. PMID: 33538338.
[5]. Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., Jemal, A., Global cancer statistics 2022: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
CA Cancer J
Clin. 2024
May-Jun;74(3):229-263. doi: 10.3322/caac.21834. Epub 2024 Apr 4. PMID:
38572751.
[6]. Saad, E. l Din, K., Loree, J. M., Sayre, E. C., Gill S., Brown, C. J., Dau, H., De Vera, M. A., 2020, Trends in the epidemiology of young-onset
colorectal cancer: A worldwide systematic review. BMC Cancer. Apr 6;20(1):288. doi:
10.1186/s12885-020-06766-9. PMID: 32252672; PMCID: PMC7137305.
[7]. Lin, S., Gao, K., Gu, S., You, L., Qian, S., Tang, M., Wang, J., Chen, K., Jin, M., 2021, Worldwide trends in cervical cancer incidence
and mortality, with predictions for the next 15 years. Cancer. Nov 1;127(21):4030-4039. doi:
10.1002/cncr.33795. Epub 2021 Aug 9. PMID: 34368955.
[8]. Global cancer burden growing, amidst mounting
need for services was originally published by The International Agency for
Research on Cancer (IARC) is the cancer agency of the World Health
Organisation- 1 February 2024
[9]. Choudhari
A. S., Mandave P. C., Deshpande M., Ranjekar P., Prakash O., Phytochemicals in
Cancer Treatment: From Preclinical Studies to Clinical Practice. Front
Pharmacol. 2020 Jan 28;10:1614. doi: 10.3389/fphar.2019.01614. Erratum in:
Front Pharmacol. 2020 Feb 28;11:175. doi: 10.3389/fphar.2020.00175. PMID:
32116665; PMCID: PMC7025531.
[10]. Pazhani J., Chanthu K., Jayaraman S.,
Varun B. R., 2023, Evaluation of salivary MMP-9 in oral squamous cell carcinoma
and oral leukoplakia using ELISA. Journal of Oral and Maxillofacial
Pathology. Oct 1;27(4):649-54.
[11]. Lee
W. L., Huang J. Y., Shyur L. F., 2013, Phytoagents for cancer management:
regulation of nucleic acid oxidation, ROS, and related mechanisms. Oxid Med
Cell Longev.;2013:925804. doi: 10.1155/2013/925804. Epub 2013 Dec 25. PMID:
24454991; PMCID: PMC3886269.
[12]. Yan, X., Xie, T., Wang, S., Wang, Z., Li, H., & Ye,
Z., 2018. Apigenin inhibits proliferation of
human chondrosarcoma cells via cell cycle arrest and mitochondrial apoptosis
induced by ROS generation-an in vitro and in vivo study. Int J Clin Exp Med,
11(3):1615-1631 www.ijcem.com /ISSN:1940-5901/IJCEM0057902.
[13]. Lu,
L., Zhao, Z., Liu, L., Gong, W., and Dong, J., 2018, Combination of baicalein
and docetaxel additively inhibits the growth of non-small cell lung cancer in
vivo. Tradit. Med. Modern Med. 01 (03), 213–218. doi: 10.1142/ S2575900018500131.
[14]. Farnsworth N. R., Akerele O., Bingel A. S., Soejarto D. D., Guo Z., 1985, Medicinal plants in therapy. Bull World Health
Organ.;63(6):965-81. PMID: 3879679; PMCID: PMC2536466.
[15]. Michael Heinrich, Joanne Barnes, Simon Gibbons,
Elizabeth M., Williamson Foreword by A. Douglas Kinghorn,
Chapter 8-Anticancer natural products, Fundamentals of pharmacognosy and
phytotherapy, First edition 2004, Second edition 2012- ISBN 978-0-7020-3388-9- © 2012 Elsevier Ltd.
[16]. Kerry
Bone,Simon Mills Forewords by Michael Dixon, Mark Blumenthal- Malignant
diseases- Herbal approaches to pathological states, Chapter 8, Principles and
Practice of Phytotherapy Modern Herbal Medicine, First
edition 2000, Second edition 2013-ISBN 978-0-443-06992-5-© 2013 Elsevier Ltd.
[17]. Bansal S., Vyas S., Bhattacharya S., Sharma M., 2013, Catechin prodrugs and analogs: a new array of
chemical entities with improved pharmacological and pharmacokinetic properties.
Nat Prod
Rep. Oct
11;30(11):1438-54. doi: 10.1039/c3np70038k. PMID: 24056761.
[18]. Gullett N. P., Ruhul Amin A. R., Bayraktar S., Pezzuto
J. M., Shin D. M., Khuri F. R., Aggarwal B. B., Surh Y. J., Kucuk O., 2010, Cancer prevention with natural compounds. Semin Oncol. Jun;37(3):258-81. doi:
10.1053/j.seminoncol.2010.06.014. PMID: 20709209.
[19]. Jayaraman, S.,
Natarajan, S. R., Ponnusamy, B., Veeraraghavan, V. P. and Jasmine, S., 2023.
Unlocking the potential of beta sitosterol: Augmenting the suppression of oral
cancer cells through extrinsic and intrinsic signalling mechanisms. The
Saudi Dental Journal, 35(8), pp.1007-1013.
[20]. Sruthi M. A., Mani G.,
Ramakrishnan M., Selvaraj J., Dental caries as a source of Helicobacter pylori
infection in children: An RT‐PCR study. International Journal of Paediatric
Dentistry. 2023 Jan;33(1):82-8.
[21]. Morgensztern
D., McLeod H. L., 2005, PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. Sep;16(8):797-803. doi: 10.1097/01.cad.0000173476.67239.3b. PMID:
16096426.
[22]. Jiri
Polivka, Filip Janku, Molecular targets for cancer therapy in the PI3K/AKT/mTOR
pathway, Pharmacology & Therapeutics, Volume 142, Issue 2, 2014, pp.
164-175, ISSN 0163-7258, https://doi.org/10.1016/j.pharmthera.2013.12.004.
[23]. Anna Prossomariti, Giulia Piazzi, Chiara Alquati, Luigi Ricciardiello, 2020,
Are Wnt/β-Catenin and PI3K/AKT/mTORC1 Distinct Pathways in Colorectal Cancer?, Cellular
and Molecular Gastroenterology and Hepatology, Volume 10, Issue 3, pp.
491-506, ISSN 2352-345X, https://doi.org/10.1016/j.jcmgh.2020.04.007.
[24]. Chen J.,
2013, Potential value and limitation of dual inhibitors of PI3K and mTOR in the
treatment of cancer. Curr Cancer Drug Targets. Feb;13(2):117-20. doi:
10.2174/1568009611313020001. PMID: 23215718.
[25]. Chen,
S., Fisher, R. C., Signs, S., Molina, L. A., Shenoy, A. K., Lopez, M. C.,
Baker, H. V., Koomen, J. M., Chen, Y., Gittleman, H., Barnholtz-Sloan, J.,
Berg, A., Appelman, H. D., & Huang, E. H., 2017, Inhibition of
PI3K/Akt/mTOR signaling in PI3KR2-overexpressing colon cancer stem cells
reduces tumor growth due to apoptosis. Oncotarget, 8(31),
50476-50488. https://doi.org/10.18632/oncotarget.9919
[26]. Mangiapane L. R., Nicotra A., Turdo A., Gaggianesi M.,
Bianca P., et al., 2022, PI3K-driven HER2 expression is a potential therapeutic
target in colorectal cancer stem cells. Gut. Jan;71(1):119-128. doi:
10.1136/gutjnl-2020-323553. Epub 2021 Jan 12. PMID: 33436496; PMCID:
PMC8666826.
[27]. Cai
Z., Ke J., He X., Yuan R., Chen Y., Wu X., Wang L., Wang J., Lan P., Wu X., 2014,
Significance of mTOR signaling and its inhibitor against cancer stem-like cells
in colorectal cancer. Ann Surg Oncol. Jan;21(1):179-88. doi:
10.1245/s10434-013-3146-8. Epub 2013 Aug 2. PMID: 23907312.
[28]. Malkomes, P., Lunger, I., Luetticke, A. et al., 2016, Selective AKT
Inhibition by MK-2206 Represses Colorectal Cancer-Initiating Stem Cells. Ann
Surg Oncol 23, 2849–2857. https://doi.org/10.1245/s10434-016-5218-z.
[29]. Chang, L., Graham, P. H., Hao, J., Bucci, J., Cozzi, P. J.,
Kearsley, J. H., Li, Y., 2014, Emerging roles of radioresistance in prostate
cancer metastasis and radiation therapy. Cancer Metastasis Rev.
Sep;33(2-3):469-96. doi: 10.1007/s10555-014-9493-5. PMID: 24445654.
[30]. Sircar, K., Yoshimoto, M., Monzon, F. A., Koumakpayi, I. H.,
Katz, R. L., Khanna, A., Alvarez, K., Chen, G., Darnel, A. D., Aprikian, A. G.,
Saad F., Bismar, T. A., Squire, J. A., 2009, PTEN genomic deletion is
associated with p-Akt and AR signalling in poorer outcome, hormone refractory
prostate cancer. J Pathol. Aug;218(4):505-13. doi: 10.1002/path.2559.
PMID: 19402094.
[31]. de Muga, S., Hernández, S., Agell, L., Salido, M., Juanpere, N., Lorenzo, M.,
Lorente, J. A., Serrano, S., Lloreta, J., 2010, Molecular alterations of EGFR
and PTEN in prostate cancer: association with high-grade and advanced-stage
carcinomas. Mod Pathol. May;23(5):703-12. doi:
10.1038/modpathol.2010.45. Epub 2010 Mar 5. PMID: 20208477.
[32]. Niraj
Kumar, Jha, Saniya Arfin, Saurabh Kuma,r Jha, Rohan Kar, Abhijit Dey, Rohit
Gundamaraju, Ghulam, M. d., Ashraf, Piyush Kumar Gupta, Sugapriya Dhanasekaran,
Mosleh Mohammad Abomughaid, Sabya Sachi Das, Sachin Kumar Singh, Kamal Dua,
Shubhadeep Roychoudhury, Dhruv Kumar, Janne Ruokolainen, Shreesh Ojha, Kavindra
Kumar Kesari, Re-establishing the comprehension of phytomedicine and
nanomedicine in inflammation-mediated cancer signaling, Seminars in Cancer
Biology, Volume 86, Part 2, 2022, pp. 1086-1104,
ISSN 1044-579X, https://doi.org/10.1016/j.semcancer.2022.02.022.
[33]. Akkoç,
Y., Berrak, Ö., Arısan, E. D., Obakan, P., Çoker-Gürkan, A., Palavan-Ünsal, N., 2015, Inhibition of PI3K signaling triggered
apoptotic potential of curcumin which is hindered by Bcl-2 through activation
of autophagy in MCF-7 cells. Biomed Pharmacother., Apr;71:161-71. doi:
10.1016/j.biopha.2015.02.029. Epub 2015 Mar 4. PMID: 25960232.
[34]. Guan F.,
Ding Y., Zhang Y., Zhou Y., Li M., Wang C., 2016, Curcumin Suppresses
Proliferation and Migration of MDA-MB-231 Breast Cancer Cells through
Autophagy-Dependent Akt Degradation. PLoS One. Jan 11;11(1):e0146553.
doi: 10.1371/journal.pone.0146553. Retraction in: PLoS One. 2023 Mar
15;18(3):e0283354. doi: 10.1371/journal.pone.0283354. PMID: 26752181; PMCID:
PMC4708990.
[35]. Ibrahim RS, Ibrahim S. S., El-Naas A., Koklesová L., Kubatka P., Büsselberg D., 2023, Could Metformin and Resveratrol Support
Glioblastoma Treatment? A Mechanistic View at the Cellular Level. Cancers
(Basel). Jun 27;15(13):3368. doi: 10.3390/cancers15133368. PMID: 37444478;
PMCID: PMC10340608.
[36]. Cháirez-Ramírez, M. H., de la Cruz-López, K. G, García-Carrancá, A., 2021, Polyphenols as Antitumor Agents Targeting Key
Players in Cancer-Driving Signaling Pathways. Front Pharmacol. Oct 20;12:710304. doi:
10.3389/fphar.2021.710304. PMID: 34744708; PMCID: PMC8565650.
[37]. Krishnan,
Reshma Poothakulath, et al. 2025, "Molecular profiling of oral epithelial
dysplasia and oral squamous cell carcinoma using next generation
sequencing." Journal of Stomatology, Oral and Maxillofacial
Surgery 126.4, 102120.
[38]. Bhullar,
K. S., Jha, A., Rupasinghe, H. P., 2015, Novel carbocyclic curcumin analog
CUR3d modulates genes involved in multiple apoptosis pathways in human
hepatocellular carcinoma cells. Chem Biol Interact., Dec 5;242:107-22.
doi: 10.1016/j.cbi.2015.09.020. Epub 2015 Sep 26. PMID: 26409325.
[39]. Zhao, Z.,
Li, C., Xi, H., Gao, Y., Xu, D., 2015, Curcumin induces apoptosis in pancreatic
cancer cells through the induction of forkhead box O1 and inhibition of the
PI3K/Akt pathway. Mol Med Rep. Oct;12(4):5415-22. doi:
10.3892/mmr.2015.4060. Epub 2015 Jul 8. PMID: 26166196.
[40]. Xu, X.,
Qin, J., Liu, W., 2014, Curcumin inhibits the invasion of thyroid cancer cells
via down-regulation of PI3K/Akt signaling pathway. Gene. Aug
10;546(2):226-32. doi: 10.1016/j.gene.2014.06.006. Epub 2014 Jun 6. PMID:
24910117.
[41]. Jiang Q.
G., Li T. Y., Liu D. N., Zhang H. T., 2014, PI3K/Akt pathway involving into
apoptosis and invasion in human colon cancer cells LoVo. Mol Biol Rep.
May;41(5):3359-67. doi: 10.1007/s11033-014-3198-2. Epub 2014 Feb 5. PMID:
24496855.
[42]. Lee, J.
H., Jang, J. Y., Park, C., Kim, B. W., Choi, Y. H., Choi B. T., Curcumin
suppresses alpha-melanocyte stimulating hormone-stimulated melanogenesis in
B16F10 cells. Int J Mol Med. 2010 Jul;26(1):101-6. PMID: 20514428.
[43]. Kim M. O.,
Lee M. H., Oi N., Kim S. H., Bae K. B., Huang Z., Kim D. J., Reddy K., Lee S. Y.,
Park S. J., Kim J. Y., Xie H., Kundu J. K., Ryoo Z. Y., Bode A. M., Surh Y. J.,
Dong Z., 2014, [6]-shogaol inhibits growth and
induces apoptosis of non-small cell lung cancer cells by directly regulating
Akt1/2. Carcinogenesis. Mar;35(3):683-91. doi:
10.1093/carcin/bgt365. Epub 2013 Nov 26. Erratum in: Carcinogenesis. 2014
May;35(5):1193. PMID: 24282290; PMCID: PMC3941745.
[44]. Sagar S., Ramani P.,
Moses S., Gheena S., Selvaraj J., 2024, Correlation of salivary cytokine IL-17A
and 1, 25 dihydroxycholecalciferol in patients undergoing orthodontic
treatment. Odontology. Feb 6:1-0.
[45]. Yasothkumar D., Ramani P.,
Jayaraman S., Ramalingam K., Tilakaratne W. M., 2024, Expression Profile of
Circulating Exosomal microRNAs in Leukoplakia, Oral Submucous Fibrosis, and
Combined Lesions of Leukoplakia and Oral Submucous Fibrosis. Head and Neck
Pathology. Mar 27;18(1):28.
[46]. Jane, D Holland, Alexandra Klaus, Alistair N Garratt, Walter Birchmeier,
2013,
Wnt signaling in stem and cancer stem cells, Current Opinion in Cell
Biology, Volume 25, Issue 2, pp. 254-264, ISSN
0955-0674, https://doi.org/10.1016/j.ceb.2013.01.004.
[47]. Wend, P., Holland, J. D., Ziebold U., Birchmeier W., 2010,
Wnt signaling in stem and cancer stem cells. Semin Cell Dev Biol.
Oct;21(8):855-63. doi: 10.1016/j.semcdb.2010.09.004. Epub 2010 Sep 15. PMID:
20837152.
[48]. Sun, J., Yan, P., Chen, Y., Chen, Y., Yang, J., Xu, G., Mao,
H., Qiu, Y., 2015, MicroRNA-26b inhibits cell proliferation and cytokine
secretion in human RASF cells via the Wnt/GSK-3β/β-catenin pathway. Diagn Pathol. Jun 19;10:72. doi:
10.1186/s13000-015-0309-x. PMID: 26088648; PMCID: PMC4472173.
[49]. Martins-Neves S. R., Corver W. E., Paiva-Oliveira D. I.,
van den Akker B. E., Briaire-de-Bruijn I. H., Bovée J. V.,
Gomes C. M., Cleton-Jansen A. M., 2016, Osteosarcoma Stem Cells Have Active
Wnt/β-catenin
and Overexpress SOX2 and KLF4. J Cell Physiol., Apr;231(4):876-86. doi:
10.1002/jcp.25179. Epub 2015 Sep 9. PMID: 26332365.
[50]. Miyoshi, Y., Iwao, K., Nagasawa, Y., Aihara, T., Sasaki, Y.,
Imaoka, S., Murata, M., Shimano, T., Nakamura, Y., 1998, Activation of the
beta-catenin gene in primary hepatocellular carcinomas by somatic alterations
involving exon 3. Cancer Res., Jun 15;58(12):2524-7. PMID: 9635572.
[51]. Huang, G. L., Zhang, W., Ren H. Y., Shen X. Y., Chen Q. X.,
Shen D. Y., Retinoid X receptor α enhances human cholangiocarcinoma growth through
simultaneous activation of Wnt/β-catenin and nuclear factor-κB pathways. 2015, Cancer Sci., Nov;106(11):1515-23.
doi: 10.1111/cas.12802. Epub 2015 Oct 7. PMID: 26310932; PMCID: PMC4714697.
[52]. Wu G., Liu A., Zhu J., Lei F., Wu S., Zhang X., Ye L., Cao
L., He S., 2015, MiR-1207 overexpression promotes cancer stem cell-like
traits in ovarian cancer by activating the Wnt/β-catenin signaling pathway. Oncotarget. Oct 6;6(30):28882-94. doi: 10.18632/oncotarget.4921. PMID: 26337084;
PMCID: PMC4745698.
[53]. Du Q., Zhang X., Cardinal J., Cao Z., Guo Z., Shao L.,
Geller D. A., 2009, Wnt/beta-catenin signaling regulates cytokine-induced human
inducible nitric oxide synthase expression by inhibiting nuclear factor-kappaB
activation in cancer cells. Cancer Res. May 1;69(9):3764-71. doi:
10.1158/0008-5472.CAN-09-0014. Epub 2009 Apr 21. PMID: 19383900.
[54]. Logan C. Y., Nusse R., 2004, The Wnt signaling pathway in
development and disease. Annu Rev Cell Dev Biol.; 20:781-810. doi:
10.1146/annurev.cellbio.20.010403.113126. PMID: 15473860.
[55]. Pandey, P., Khan, F., Seifeldin, S. A., Alshaghdali, K., Siddiqui, S., Abdelwadoud, M. E., Vyas, M., Saeed, M., Mazumder, A., Saeed, A., 2023, Targeting Wnt/β-Catenin Pathway by Flavonoids: Implication
for Cancer Therapeutics. Nutrients. Apr 26;15(9):2088. doi: 10.3390/nu15092088.
PMID: 37432240; PMCID: PMC10181252.
[56]. Mukherjee, S., Mazumdar, M., Chakraborty, S. et al., 2014, Curcumin inhibits breast cancer stem cell migration by
amplifying the E-cadherin/β-catenin negative feedback loop. Stem Cell Res Ther 5,
116, https://doi.org/10.1186/scrt506.
[57]. Somers-Edgar T. J., Taurin S., Larsen L., Chandramouli A.,
Nelson M. A., Rosengren R. J., 2011, Mechanisms for the activity of
heterocyclic cyclohexanone curcumin derivatives in estrogen receptor negative
human breast cancer cell lines. Invest New Drugs. Feb;29(1):87-97. doi:
10.1007/s10637-009-9339-0. Epub 2009 Oct 9. PMID: 19816657.
[58]. Lu, Y., Wei, C., Xi, Z., 2014, Curcumin
suppresses proliferation and invasion in non-small cell lung cancer by
modulation of MTA1-mediated Wnt/β-catenin pathway. In Vitro Cell Dev Biol Anim.
Oct;50(9):840-50. doi: 10.1007/s11626-014-9779-5. Epub 2014 Jun 18. PMID:
24938356.
[59]. Lai C. S., Wu J. C., Yu S. F., Badmaev V., Nagabhushanam K.,
Ho C. T., Pan M. H., 2011, Tetrahydrocurcumin is more effective than curcumin
in preventing azoxymethane-induced colon carcinogenesis. Mol Nutr Food Res.
Dec;55(12):1819-28. doi: 10.1002/mnfr.201100290. Epub 2011 Sep 2. PMID:
21887819.
[60]. Fathima
J. S., Jayaraman S., Sekar R., Syed N. H., 2024, The role of MicroRNAs in the
diagnosis and treatment of oral premalignant disorders. Odontology. Apr
15:1-0.
[61]. Kunnumakkara A. B., Bordoloi D., Harsha C., Banik K., Gupta
S. C., Aggarwal B. B., 2017, Curcumin mediates anticancer effects by modulating
multiple cell signaling pathways. Clin Sci (Lond). Jul
5;131(15):1781-1799. doi: 10.1042/CS20160935. PMID: 28679846.
[62]. Zhang, Y., Li Q., Zhou D., Chen H., 2013, Genistein, a soya
isoflavone, prevents azoxymethane-induced up-regulation of WNT/β-catenin signalling
and reduces colon pre-neoplasia in rats. Br J Nutr. Jan 14;109(1):33-42.
doi: 10.1017/S0007114512000876. Epub 2012 Apr 3. PMID: 22716201.
[63]. Hsiao Y. C., Peng S. F., Lai K. C., Liao C. L., Huang Y. P.,
Lin C. C., Lin M. L., Liu K. C., Tsai C. C., Ma Y. S., Chung J. G., 2019,
Genistein induces apoptosis in vitro and has antitumor activity against human
leukemia HL-60 cancer cell xenograft growth in vivo. Environ Toxicol.
Apr;34(4):443-456. doi: 10.1002/tox.22698. Epub 2019 Jan 7. PMID: 30618158.

