Evaluation of nCD64 in Patients with Periodontitis
Abstract:
Periodontitis causes tissue destruction and tooth loss if untreated.
Neutrophil CD64 (nCD64), a biomarker will help in the early and precise
diagnosis of inflammation. Thus, the aim of this study is to evaluate the
potential of neutrophil nCD64 as a diagnostic marker by looking at the
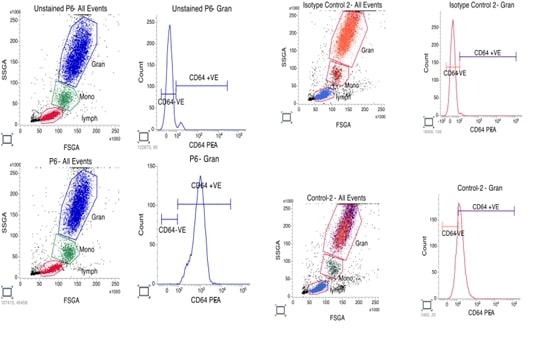
expression levels of nCD64 in people with periodontitis. This study involved 12
participants comprising of 6 healthy controls and 6 of patients with
periodontitis. nCD64 levels were measured on acquired blood samples using flow
cytometry. Mean fluorescence intensity (MFI) of nCD64 was compared between the
two groups. When compared to healthy controls, patients with periodontitis had
noticeably higher nCD64 MFI levels. In a patient, the greatest nCD64 MFI level
was 861, whereas in a control, the lowest level was 19. Comorbid conditions
like diabetes did not always correspond with elevated nCD64 levels, suggesting
that periodontitis severity was the main factor affecting nCD64 expression. The
current study suggests nCD64 as a useful biomarker for identifying periodontal
inflammation, which helps with the timely and precise diagnosis of
periodontitis. Future studies are necessary to corroborate these results using
more extensive and heterogeneous groups.
References:
[1].
Mony,
U., Sanju, S., Jain, P., Sugavanan, K., Sebastian, A., Theertha, M.,
Sidharthan, N., Varma, P. K., 2019, Detection of dysregulated host response by
flow cytometry may pre-empt early diagnosis of sepsis after cardiac surgery. Blood,
134, 4863.
[2]. Ng, P. C, Li, G., Chui, K. M., Chu, W. C., Li, K., Wong, R. P,
Chik, K. W, Wong, E., Fok, T. F., 2004, Neutrophil CD64 is a sensitive
diagnostic marker for early-onset neonatal infection. Pediatr Res,
56(5), 796-803.
[3].
Davis,
B. H., Olsen, S. H., Ahmad, E., Bigelow, N. C., 2006, Neutrophil CD64 is an
improved indicator of infection or sepsis in emergency department patients. Arch
Pathol Lab Med, 130(5), 654-61.
[4].
Naiff,
P. F., Kuckelhaus, S. A., Couto, S., Oliveira, M., Santiago, L. M., Cascaes, A.
C., Silva, L. F., Oliveira, L. A., Grisi, D. C., Carneiro, V. M., Guimarães, M.
D. C. M., 2021, Phagocytic activity of monocytes and neutrophils in patients
with periodontitis, whether or not associated to type 2 diabetes. Acta
Odontol Latinoam, 34(3), 201-213.
[5].
Magán-Fernández,
A., Rasheed, Al-Bakri, S. M., O'Valle, F., Benavides-Reyes, C., Abadía-Molina,
F., Mesa, F., 2020, Neutrophil Extracellular Traps in Periodontitis. Cells,
9(6), 1494.
[7].
Doganyigit,
Z., Eroglu, E., Akyuz, E., 2022, Inflammatory mediators of cytokines and
chemokines in sepsis: From bench to bedside. Human & experimental
toxicology, 41, 09603271221078871.
[8].
Liu,
Q., Gao, Y., Yang, T., Zhou, Z., Lin, K., Zhang, W., Li, T., Lu, Y., Shao, L.,
Zhang, W., 2022, nCD64 index as a novel inflammatory indicator for the early
prediction of prognosis in infectious and non-infectious inflammatory diseases:
An observational study of febrile patients. Front Immunol, 13, 905060.
[9].
Wang,
X., Li, Z. Y., Zeng, L., Zhang, A. Q., Pan, W., Gu, W., Jiang, J. X., 2015, Neutrophil
CD64 expression as a diagnostic marker for sepsis in adult patients: a
meta-analysis. Crit Care, 19(1), 245.
[10]. Hirschfeld, J., 2020, Neutrophil Subsets in Periodontal
Health and Disease: A Mini Review. Front Immunol, 10, 3001.
[11]. Oppegaard, O., Skodvin, B., Halse, A. K., Langeland, N.,
2013, CD64 as a potential biomarker in septic arthritis. BMC Infect Dis,
13:278.
[12]. Xing, W., Wang, Y., Liu, J., Pei, J., Yu, C., 2023, Role of
interleukins in the detection of neonatal sepsis: a network meta-analysis. Frontiers
in Pediatrics, 11, 1267777.
[13]. Cid, J., García-Pardo, G., Aguinaco, R., Sánchez, R.,
Llorente, A., 2011, Neutrophil CD64: diagnostic accuracy and prognostic value
in patients presenting to the emergency department. Eur J Clin Microbiol
Infect Dis, 30(7), 845-52.
[14]. Allen, E., Bakke, A. C., Purtzer, M. Z., Deodhar, A., 2002,
Neutrophil CD64 expression: distinguishing acute inflammatory autoimmune
disease from systemic infections. Annals of the rheumatic diseases, 61(6),
522-5.
[15]. Bakke, A. C., Allen, E., Purtzer, M. Z., Deodhar, A., 2001,
Neutrophil CD64 expression distinguishing acute inflammatory autoimmune disease
from systemic infections. Clinical and Applied Immunology Reviews, 1(5),
267-75.
[16]. Sanju,
S., Jain, P., Vishnu Priya, V., Varma, P. K., Mony, U., 2023, Quantitation of
mHLA- DR and nCD64 by Flow Cytometry to Study Dysregulated Host Response: The
Use of QuantiBRITETM PE Beads and Its Stability. Appl Biochem Biotechnol,
195(9),5747-5752.
[17]. Agnes,
S., S., Sanju, Jain, P., Varma, P. K., Mony, U., 2021, Non-classical monocytes
and its potential in diagnosing sepsis post cardiac surgery. International
Immunopharmacology, 99,108037
[18]. Hassan, U., Ghonge, T., Reddy, Jr. B., Patel, M.,
Rappleye, M., Taneja, I., Tanna, A., Healey, R., Manusry, N., Price, Z.,
Jensen, T., 2017, A point-of-care microfluidic biochip for quantification of
CD64 expression from whole blood for sepsis stratification. Nature communications, 8(1),15949
[19]. Liu, Q., Gao, Y., Yang, T., Zhou, Z., Lin, K.,
Zhang, W., Li, T., Lu, Y., Shao, L., Zhang W., 2022, nCD64 index as a novel
inflammatory indicator for the early prediction of prognosis in infectious and
non-infectious inflammatory diseases: An observational study of febrile
patients. Front
Immunol,
13,905060.
[20]. Ghosh, P. S., Singh, H., Azim, A., Agarwal, V.,
Chaturvedi, S., Saran, S., Mishra, P., Gurjar, M., Baronia, A. K, Poddar, B.,
Singh, R. K, Mishra, R., 2018, Correlation of Neutrophil CD64 with Clinical
Profile and Outcome of Sepsis Patients during Intensive Care Unit Stay. Indian J Crit Care Med, 22(8),569-574.
[21]. Renu, K., Gopalakrishnan, A.V. and Madhyastha,
H., 2024. Is periodontitis triggering an inflammatory response in the liver,
and does this reaction entail oxidative stress?. Odontology, pp.1-14.
[22].
Thomas,
J.T., Joseph, B., Varghese, S., Kamalasanan Vijayakumari, B., Sorsa, T.,
Mauramo, M., Anil, S. and Waltimo, T., 2024. Salivary advanced glycated end
products, their receptors, and aMMP‐8 in periodontitis patients with varying
glycemic levels: A cross‐sectional study. Journal of
Periodontology.
[23].
Uppin,
R.B., Varghese, S.S., Baseer, M.A., AlMugeiren, O.M., Mubaraki, S. and
Alsaffan, A.D., 2024. Knowledge and Awareness of Metabolic Syndrome and its
Relationship with Periodontal Disease among Dental Practitioners in Riyadh
City, Saudi Arabia: A Cross-Sectional Study. Journal of
International Oral Health, 16(6), pp.487-497.
[24].
Yadalam,
P.K., Barbosa, F.T., Natarajan, P.M. and Ardila, C.M., 2024. Graph Neural
Networks-Based Prediction of Drug Gene Interactions of RTK-VEGF4 Receptor
Family in Periodontal Regeneration. Journal of Clinical and Experimental Dentistry, 16(12),
p.e1454.
[25].
Priyangha,
P.T., Kshirsagar, J.T. and Kalaiselvan, D., 2024. Exploring Serum Ceruloplasmin
Dynamics in Stage II Periodontitis: Pre-and PostPhase I Therapy
Assessment. Journal of the International Clinical Dental
Research Organization, 16(2), pp.135-140.
[26]. Priya, V., Keerthivasan, S. and Kaviyarasi, R.,
Determining the Dual Effect of Mirabegron on Anticancer Mechanism and Brown
Adipose Tissue Activation-An in-silico Approach.
[27]. Juvairiya
Fathima, A., Renu, K., Priya, V.V., Gayathri, R. and Kavitha, S., Determining
the Role of Caffeic Acid on Lipogenic Regulators: An In-Silico Approach.
[28]. Aarthi, L., Renu, K., Priya, V.V., Gayathri, R.
and Kavitha, S., Molecular Docking Analysis of Epigallocatechin 3-Gallate
[EGCG] on Fatty Acids and Carnitine Transporters Family.
Hirshasri, A.G., Renu, K., Priya, V.V., Gayathri, R. and Kavitha, S., The Effect of Aspalathin on SMAD2, SMAD3, TGF-β-A Major Contributor of Inflammation–An In-silico Approach

