Molecular Mechanisms Underlying the Anticancer Activity of Chrysin Through p53 Tumor Suppressor in HepG2 Cell Lines
Abstract:
Chrysin,
a natural flavonoid found in passionflower, honey, and propolis, is gaining
attention for its antioxidant, anti-inflammatory, and anticancer properties.
This study evaluates chrysin’s anticancer efficacy against HepG2 liver cancer
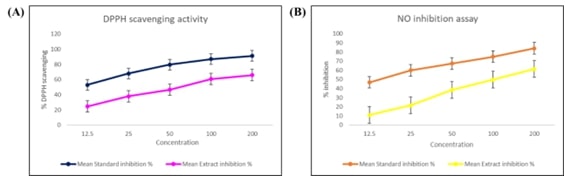
cells. We assessed its antioxidant potential using DPPH and nitric oxide
scavenging assays, which revealed significant, concentration-dependent radical
scavenging activity, emphasizing chrysin’s strong antioxidant properties. These
effects are likely to reduce oxidative stress, a factor that promotes cancer
cell proliferation and survival. Cytotoxicity was measured with the MTT assay,
and gene expression analysis through RT-qPCR showed that chrysin upregulated
pro-apoptotic genes such as Bax, Caspase 3, and Caspase 9, while downregulating
the anti-apoptotic gene Bcl-2. Notably, chrysin also increased the expression
of the tumor suppressor gene p53, essential for cell cycle regulation and
apoptosis in response to stress and DNA damage. Molecular docking studies were
performed to investigate chrysin’s interactions with key apoptotic proteins.
The docking results showed strong binding affinities between chrysin and Bax,
Bcl-2, Caspase 3, Caspase 9, and p53. Particularly high binding affinities with
Caspase 9 and p53 suggest that chrysin may effectively trigger the intrinsic
apoptotic pathway, leading to cancer cell death. The interaction with p53 is
significant as it may stabilize and activate p53, enhancing the transcription
of pro-apoptotic genes. These findings highlight chrysin's potential as a
therapeutic agent for liver cancer, primarily through the p53-mediated
apoptotic pathway. While these in vitro results are promising, further in vivo
studies and clinical trials are necessary to confirm chrysin’s efficacy and
safety in a clinical setting.
References:
[1].
Llovet, J. M., Kelley, R.
K., Villanueva, A., Singal, A. G., Pikarsky, E., Roayaie, S., Lencioni, R.,
Koike, K., Zucman-Rossi, J., & Finn, R. S., 2021, Hepatocellular
carcinoma. Nature Reviews. Disease Primers, 7(1), 6. https://doi.org/10.1038/s41572-020-00240-3
[2].
Khemlina, G., Ikeda, S.,
& Kurzrock, R., 2017, The biology of Hepatocellular carcinoma: implications
for genomic and immune therapies. Molecular Cancer, 16(1),
149. https://doi.org/10.1186/s12943-017-0712-x
[3].
Hong, W., Zhang, Y., Wang,
S., Zheng, D., Hsu, S., Zhou, J., Fan, J., Zeng, Z., Wang, N., Ding, Z., Yu,
M., Gao, Q., & Du, S., 2024, Deciphering the immune modulation through deep
transcriptomic profiling and therapeutic implications of DNA damage repair
pattern in hepatocellular carcinoma. Cancer Letters, 582,
216594. https://doi.org/10.1016/j.canlet.2023.216594
[4].
Zheng, J., Chen, J., Wang,
S., Yang, D., & Zhou, P., 2024, Genomic and immune landscape in
hepatocellular carcinoma: Implications for personalized therapeutics. Environmental
Toxicology, 39(3), 1601–1616. https://doi.org/10.1002/tox.24062
[5].
Wang, Y., & Chen, H., 2023,
Protein glycosylation alterations in hepatocellular carcinoma: function and
clinical implications. Oncogene, 42(24), 1970–1979. https://doi.org/10.1038/s41388-023-02702-w
[6].
Mo, Z., Liu, D., Rong, D.,
& Zhang, S., 2021, Hypoxic
Characteristic in the Immunosuppressive Microenvironment of Hepatocellular
Carcinoma. Frontiers in Immunology, 12, 611058. https://doi.org/10.3389/fimmu.2021.611058
[7].
Mani, R., & Natesan, V.,
2018, Chrysin: Sources, beneficial pharmacological activities, and molecular
mechanism of action. Phytochemistry, 145, 187-196.
[8].
Hatano, T., Yasuhara, T.,
Yoshihara, R., Agata, I., Noro, T., & OKUDA, T., 1990, Effects of
interaction of tannins with co-existing substances. VII.: inhibitory effects of
tannins and related polyphenols on xanthine oxidase. Chemical and
Pharmaceutical Bulletin, 38(5), 1224-1229.
[9]. Jayaraman, S., Natararaj, S., & Veeraraghavan,
V. P., 2024, Hesperidin inhibits oral cancer cell growth via apoptosis and
inflammatory signaling-mediated mechanisms: Evidence from in vitro and in
silico analyses. Cureus, 16(2).
[10]. Jayaraman, S., Natarajan, S. R., Veeraraghavan, V.
P., & Jasmine, S., 2023, Unveiling the anti-cancer mechanisms of
calotropin: Insights into cell growth inhibition, cell cycle arrest, and
metabolic regulation in human oral squamous carcinoma cells (HSC-3). Journal
of Oral Biology and Craniofacial Research, 13(6), 704-713.
[11]. Jayaraman, S., Veeraraghavan, V. P., Natarajan, S.
R., & Jasmine, S., 2024, Exploring the therapeutic potential of curcumin in
oral squamous cell carcinoma (HSC-3 cells): Molecular insights into
hypoxia-mediated angiogenesis. Pathology-Research and Practice, 254,
155130.
[12]. Roy, J. R., Janaki, C. S., Jayaraman, S.,
Periyasamy, V., Balaji, T., Vijayamalathi, M., & Veeraraghavan, V. P., 2022,
Carica papaya reduces muscle insulin resistance via IR/GLUT4 mediated signaling
mechanisms in high fat diet and streptozotocin-induced type-2 diabetic rats. Antioxidants,
11(10), 2081.
[13]. Roy, J. R., Janaki, C. S., Jayaraman, S.,
Periyasamy, V., Balaji, T., Vijayamalathi, M., & Prasad, M., 2023, Carica
papaya reduces high fat diet and streptozotocin-induced development of
inflammation in adipocyte via IL-1β/IL-6/TNF-α mediated signaling mechanisms in
type-2 diabetic rats. Current Issues in Molecular Biology, 45(2), 852.
[14]. Deenadayalan, A., Subramanian, V., Paramasivan, V.,
Veeraraghavan, V. P., Rengasamy, G., Coiambatore Sadagopan, J., &
Jayaraman, S., 2021, Stevioside attenuates insulin resistance in skeletal
muscle by facilitating IR/IRS-1/Akt/GLUT 4 signaling pathways: An in vivo and
in silico approach. Molecules, 26(24), 7689.
[15]. Narasimhan, A., Sampath, S., Jayaraman, S., &
Karundevi, B., 2013, Estradiol favors glucose oxidation in gastrocnemius muscle
through modulation of insulin signaling molecules in adult female rats. Endocrine
Research, 38(4), 251-262.
[16]. Roy, J. R., Janaki, C. S., Jayaraman, S.,
Periyasamy, V., Balaji, T., Vijayamalathi, M., & Veeraraghavan, V. P., 2022,
Effect of Carica papaya on IRS-1/Akt signaling mechanisms in
High-Fat-Diet–Streptozotocin-Induced type 2 diabetic experimental rats: A
mechanistic approach. Nutrients, 14(19), 4181.
[17]. Perumal, S., Langeshwaran, K., Selvaraj, J.,
Ponnulakshmi, R., Shyamaladevi, B., & Balasubramanian, M. P., 2018, Effect
of diosmin on apoptotic signaling molecules in N-nitrosodiethylamine-induced
hepatocellular carcinoma in experimental rats. Molecular and Cellular
Biochemistry, 449, 27-37.
[18]. Devarajan, N., Jayaraman, S., Mahendra, J.,
Venkatratnam, P., Rajagopal, P., Palaniappan, H., & Ganesan, S. K., 2021, Berberine—A
potent chemosensitizer and chemoprotector to conventional cancer therapies. Phytotherapy
Research, 35(6), 3059-3077.
[19]. Selvaraj, J., Sathish, S., Mayilvanan, C., &
Balasubramanian, K., 2013, Excess aldosterone-induced changes in insulin
signaling molecules and glucose oxidation in gastrocnemius muscle of adult male
rat. Molecular and Cellular Biochemistry, 372, 113-126.
[20]. Venditto, V. J., & Simanek, E. E., 2010, Cancer therapies utilizing the camptothecins: A review of the in vivo literature. Molecular Pharmaceutics, 7(2), 307–349. https://doi.org/10.1021/mp900243b

