Myricetin Anti-diabetic Activity in 3T3 Cells Targeting the Nrf2-Keap Signaling Cascade
Abstract:
The study
was aimed at assessing the effects of Myricetin, a potent anti-cancer compound,
targets the NRF2-Keap1 pathway in 3T3-L1 fibroblast cells, which is crucial in
cancer progression, cell growth, and metastasis. Various assays have
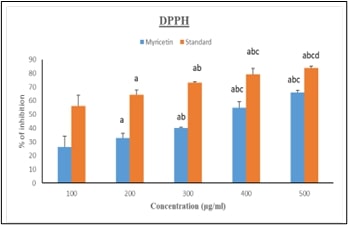
demonstrated myricetin's therapeutic potential. Antioxidant properties,
confirmed by the DPPH assay, show dose-dependent free radical inhibition, with
myricetin achieving 69.98% inhibition at 500 μg/ml (p<0.001).
Anti-inflammatory assays reveal significant reductions in inflammatory markers,
with inhibition rising to 74.75% at the same concentration (p<0.001). Gene
expression studies highlight myricetin's impact on key components of the
NRF2/Keap1 pathway, essential for cancer cell survival. In 3T3-L1 cells treated
with myricetin, notable changes were observed in mRNA expression levels: IR
(0.95±0.05, p<0.001), IL-1β (0.96±0.04, p<0.002), Keap1 (0.9±0.04,
p<0.001), Glut4 (0.6±0.04, p<0.002), NRF2 (0.96±0.4, p<0.001), and
NFκB (0.94±0.05, p<0.001). These findings suggest myricetin disrupts
critical pathways, contributing to reduced inflammation and potential cancer
inhibition. The MTT assay further indicates no cytotoxicity after 48 hours,
supporting its safety profile. Molecular docking studies reveal strong binding
affinities of myricetin to key pathway components, with Keap1 showing the
highest affinity (-9.7 kcal/mol), followed by IR (-8 kcal/mol), NFκB (-6.9
kcal/mol), and NRF2 (-6.9 kcal/mol). IL-1β and Glut4 showed affinities of -7.1
and -7.2 kcal/mol, respectively, reinforcing myricetin's role in modulating the
NRF2/Keap1 pathway. In summary, myricetin’s antioxidant, anti-inflammatory, and
gene-modulatory activities, combined with its strong molecular interactions,
position it as a promising therapeutic agent for both cancer and diabetes by
modulating key cellular pathways.
References:
[1].
Narasimhan,
A., Sampath, S., Jayaraman, S., & Karundevi, B., 2013, Estradiol favors
glucose oxidation in gastrocnemius muscle through modulation of insulin
signaling molecules in adult female rats. Endocrine Research, 38(4),
251-262.
[2].
Roy,
J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi,
M., & Veeraraghavan, V. P., 2022, Effect of Carica papaya on IRS-1/Akt
signaling mechanisms in high-fat-diet-streptozotocin-induced type 2 diabetic
experimental rats: A mechanistic approach. Nutrients, 14(19), 4181.
[3].
Perumal,
S., Langeshwaran, K., Selvaraj, J., Ponnulakshmi, R., Shyamaladevi, B., &
Balasubramanian, M. P., 2018, Effect of diosmin on apoptotic signaling
molecules in N-nitrosodiethylamine-induced hepatocellular carcinoma in
experimental rats. Molecular and Cellular Biochemistry, 449, 27-37.
[4].
Devarajan,
N., Jayaraman, S., Mahendra, J., Venkatratnam, P., Rajagopal, P., Palaniappan,
H., & Ganesan, S. K., 2021, Berberine—A potent chemosensitizer and
chemoprotector to conventional cancer therapies. Phytotherapy Research,
35(6), 3059-3077.
[5].
Jayaraman,
S., Natararaj, S., & Veeraraghavan, V. P., 2024, Hesperidin inhibits oral
cancer cell growth via apoptosis and inflammatory signaling-mediated
mechanisms: Evidence from in vitro and in silico analyses. Cureus,
16(2).
[6].
Roy,
J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi,
M., & Veeraraghavan, V. P., 2022, Carica papaya reduces muscle insulin
resistance via IR/GLUT4 mediated signaling mechanisms in high fat diet and
streptozotocin-induced type-2 diabetic rats. Antioxidants, 11(10), 2081.
[7].
Roy,
J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi,
M., & Prasad, M., 2023, Carica papaya reduces high fat diet and
streptozotocin-induced development of inflammation in adipocyte via
IL-1β/IL-6/TNF-α mediated signaling mechanisms in type-2 diabetic rats. Current
Issues in Molecular Biology, 45(2), 852.
[8].
Indu,
S., Vijayalakshmi, P., Selvaraj, J., & Rajalakshmi, M., 2021, Novel
triterpenoids from Cassia fistula stem bark depreciates STZ-induced detrimental
changes in IRS-1/Akt-mediated insulin signaling mechanisms in type-1 diabetic
rats. Molecules, 26(22), 6812.
[9].
Gupta,
G., Siddiqui, M. A., Khan, M. M., Ajmal, M., Ahsan, R., Rahaman, M. A., &
Khushtar, M., 2020, Current pharmacological trends on myricetin. Drug
Research, 70(10), 448-454.
[10].
Semwal,
D. K., Semwal, R. B., Combrinck, S., & Viljoen, A., 2016, Myricetin: A
dietary molecule with diverse biological activities. Nutrients, 8(2),
90.
[11].
Afroze,
N., Pramodh, S., Hussain, A., Waleed, M., & Vakharia, K., 2020, A review on
myricetin as a potential therapeutic candidate for cancer prevention. 3
Biotech, 10(5), 211.
[12].
Hu,
Y., Jiang, Q., Zhai, X., Liu, L., & Hong, Y., 2023, Screening and
validation of the optimal panel of reference genes in colonic epithelium and
relative cancer cell lines. Scientific Reports, 13(1), 17777.
[13].
Bhat,
I. A., Naykoo, N. A., Qasim, I., Ganie, F. A., Yousuf, Q., Bhat, B. A., Rasool,
R., Aziz, S. A., & Shah, Z. A., 2014, Association of interleukin 1 beta
(IL-1β) polymorphism with mRNA expression and risk of non-small cell lung
cancer. Meta gene, 2, 123–133.
[14].
Singh,
A., Misra, V., Thimmulappa, R. K., Lee, H., Ames, S., Hoque, M. O., Herman, J.
G., Baylin, S. B., Sidransky, D., Gabrielson, E., Brock, M. V., & Biswal,
S., 2006, Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS
Medicine, 3(10), e420.
[15].
Xiong,
L., Xie, J., Song, C., Liu, J., Zheng, J., Liu, C., Zhang, X., Li, P., &
Wang, F., 2015, The Activation of Nrf2 and Its Downstream Regulated Genes
Mediates the Antioxidative Activities of Xueshuan Xinmaining Tablet in Human
Umbilical Vein Endothelial Cells. Evidence-based complementary and alternative medicine:
eCAM, 2015, 187265.
[16].
Neralla,
M., Preethi, A., Selvakumar, S. C., & Sekar, D., 2023, Expression levels of
microRNA-7110 in oral squamous cell carcinoma. Minerva Dental and Oral Science, 73(3), 155–160.
[17].
Lalit,
H., 2023, Preparation, Characterization, and Evaluation of Cytotoxic Activity
of a Novel Titanium Dioxide Nanoparticle-infiltrated Orthodontic Adhesive: An
In Vitro Study. World Journal of
Dentistry, 14(10),
882-887.
[18].
Smith,
S. M., Lyu, Y. L., & Cai, L., 2014, NF-κB affects proliferation and
invasiveness of breast cancer cells by regulating CD44 expression. PloS One,
9(9), e106966.
[19].
Arponen,
M., Jalava, N., Widjaja, N., & Ivaska, K. K., 2022, Glucose transporters
GLUT1, GLUT3, and GLUT4 have different effects on osteoblast proliferation and
metabolism. Frontiers in Physiology, 13, 1035516.
[20].
Bae,
T., Hallis, S. P., & Kwak, M. K., 2024, Hypoxia, oxidative stress, and the
interplay of HIFs and NRF2 signaling in cancer. Experimental & Molecular
Medicine, 56(3), 501–514. https://doi.org/10.1038/s12276-024-01180-8.
[21].
Uruno,
A., Yagishita, Y., & Yamamoto, M., 2015, The Keap1-Nrf2 system and diabetes
mellitus. Archives of Biochemistry and Biophysics, 566, 76–84. https://doi.org/10.1016/j.abb.2014.12.012.
[22].
An,
Y., Xu, B. T., Wan, S. R., Ma, X. M., Long, Y., Xu, Y., & Jiang, Z. Z.,
2023, The role of oxidative stress in diabetes mellitus-induced vascular
endothelial dysfunction. Cardiovascular Diabetology, 22(1), 237. https://doi.org/10.1186/s12933-023-01965-7.
[23]. Gopalakrishnan, U., Murthy, R. T., Felicita, A. S., Alshehri, A., Awadh, W., Almalki, A., & Patil, S., 2023, Sulfate-reducing bacteria in patients undergoing fixed orthodontic treatment. International Dental Journal, 73(2), 274-279.

