Molecular Docking, Drug-Likeness and Toxicity Prediction to Determine the Role of the Taxifolin on Neurological Diseases
Abstract:
Through an in-silico approach, the effects of
taxifolin on tau, alpha 2 macroglobulin (A2M) and alpha 1 anti-chymotrypsin
(ACT), proteins are investigated in relation to neurological disorders. In
order to facilitate protein interaction, the ligand taxifolin (extracted from
the PubChem Database) and protein receptor molecules (extracted from the PDB)
are prepared, converted to PDBQT format, and uploaded in an auto dock. The
effects of taxifolin on tau protein, ACT, and A2M are still being studied, but
the preliminary findings are promising. These interactions suggest that
taxifolin may have a complex role in controlling neuroinflammation,
proteostasis, and neurodegeneration in neurological illnesses due to their
substantial binding affinity for tau, ACT, and A2M protein. Taxifolin shows
promise as a therapy for neurological disorders by targeting tau protein, ACT,
and A2M. Lipinski's Rule states that Taxifolin administered orally should not
violate more than one condition. Taxifolin examined was in category IV, which
is under the dosage of 300 < LD50 = 2000 mg/kg. A significant root mean
square value was 0.000, with a docking score of -7.2 for alpha 1
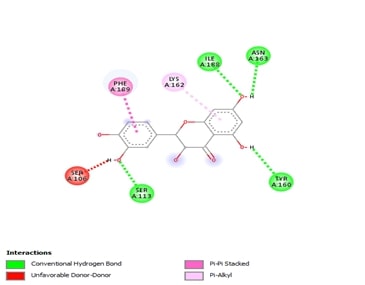
antichymotrypsin and taxifolin. Interactions in 2D and 3D include conventional
hydrogen bonds, carbon-hydrogen bonds, and unfavourable donor-donor
interactions. The chosen root mean square value was associated with a
significant docking score of -10.2 for alpha2 macroglobulin and taxifolin. The
docking score for alpha 2 macroglobulin in a two-dimensional structure, emphasising
pi-donor bonds, unfavourable donor-donor interactions, and conventional
carbon-hydrogen connections. According to these findings, taxifolin has a
significant affinity for alpha 2 macroglobulin. Tau had a high root mean square
value and a docking score of -7.5 with tau protein. Tau's 2D and 3D structures
contain pi-alkyl contacts, pi-stacking interactions, unfavourable donor-donor
interactions, and carbon-hydrogen bonds. Its ability to raise A2M activity,
decrease ACT expression, and stop tau protein aggregation suggests a variety of
potential neuroprotective benefits.
References:
[1]. Topal, F., Nar, M., Gocer, H., Kalin, P., Kocyigit, U. M., Gülçin, İ.,
& Alwasel, S. H., 2016, Antioxidant Activity of Taxifolin: an Activity–Structure
Relationship. Journal of Enzyme Inhibition and Medicinal Chemistry,
31(4), 674-683, http://dx.doi.org/10.3109/14756366.2015.1057723.
[2].
Harborne, J. B., and Williams, C. A.,
2000, Advances in Flavonoid Research Since 1992. Phytochemistry, 55(6),
481-504, http://dx.doi.org/10.1016/s0031-9422(00)00235-1.
[3].
Parameswari,
R. P., and Lakshmi, T., 2022, Microalgae as a Potential Therapeutic Drug
Candidate for Neurodegenerative Diseases. Journal of Biotechnology, 358,
128-139. http://dx.doi.org/10.1016/j.jbiotec.2022.09.009.
[4].
Kumar,
S., and Pandey, A. K., 2013, Chemistry and Biological Activities of Flavonoids:
An Overview. The Scientific World Journal, 2013(1), p.162750, http://dx.doi.org/10.1155/2013/162750.
[5].
Zhang,
C., Zhan, J., Zhao, M., Dai, H., Deng, Y., Zhou, W., and Zhao, L., 2019,
Protective Mechanism of Taxifolin for Chlorpyrifos Neurotoxicity in BV2 Cells. Neurotoxicology,
74, 74-80, http://dx.doi.org/10.1016/j.neuro.2019.05.010.
[6].
Sato,
M., Murakami, K., Uno, M., Ikubo, H., Nakagawa, Y., Katayama, S., Akagi, K. I.,
and Irie, K., 2013, Structure-activity Relationship for (+)-Taxifolin Isolated from
Silymarin as an Inhibitor of Amyloid β Aggregation. Bioscience, Biotechnology,
and Biochemistry, 77(5), 1100-1103, https://academic.oup.com/bbb/article-pdf/77/5/1100/35045235/bbb1100.pdf.
[7].
Sato,
M., Murakami, K., Uno, M., Nakagawa, Y., Katayama, S., Akagi, K. I., Masuda,
Y., Takegoshi, K., and Irie, K., 2013, Site-specific Inhibitory mechanism for Amyloid
β42 Aggregation by Catechol-type Flavonoids Targeting the Lys Residues. Journal
of Biological Chemistry, 288(32), 23212-23224, http://dx.doi.org/10.1074/jbc.M113.464222.
[8].
Inoue,
T., Saito, S., Tanaka, M., Yamakage, H., Kusakabe, T., Shimatsu, A., Ihara, M.,
and Satoh-Asahara, N., 2019, Pleiotropic Neuroprotective Effects of Taxifolin in
Cerebral Amyloid Angiopathy. Proceedings of the National Academy of
Sciences, 116(20), 10031-10038, http://dx.doi.org/10.1073/pnas.1901659116.
[9].
Licastro,
F., Mallory, M., Hansen, L. A., and Masliah, E., 1998, Increased levels of α-1-Antichymotrypsin
in Brains of Patients with Alzheimer's Disease Correlate with Activated
Astrocytes and are Affected by APOE 4 Genotype. Journal of Neuroimmunology,
88(1-2), 105-110, http://dx.doi.org/10.1016/S0165-5728(98)00096-4.
[10]. Abraham,
C. R., Selkoe, D. J., and Potter, H., 1988, Immunochemical Identification of
the Serine Protease Inhibitor α1-Antichymotrypsin in the Brain Amyloid Deposits
of Alzheimer's disease. Cell, 52(4), 487-501, http://dx.doi.org/10.1016/0092-8674(88)90462-X.
[11]. Tyagi, E., Fiorelli, T., Norden, M., and
Padmanabhan, J., 2013, Alpha 1‐Antichymotrypsin, an Inflammatory Protein
Overexpressed in the Brains of Patients with Alzheimer’s Disease, Induces Tau
Hyperphosphorylation through c‐Jun N‐Terminal Kinase Activation. International
Journal of Alzheimer’s Disease, 2013(1), p.606083, https://doi.org/10.1155/2013/606083.
[12]. Souparnika. V., Karthik G. M., and Vidya.
S., 2023, Antioxidant Activity Of L - Theanine On Cadmium Induced Oxidative
Stress Mediated Neurodegeneration - An in
vivo Analysis. Journal of Population Therapeutics and Clinical
Pharmacology, 29(02), pp. 123–130, https://doi.org/10.47750/jptcp.2022.952.
[13]. Veeraraghavan, V. P., Jayaraman, S., Rengasamy, G., Mony, U., Ganapathy, D.
M., Geetha, R. V., and Sekar, D., 2021, Deciphering the role of microRNAs in
neuroblastoma. Molecules, 27(1), p.99, http://dx.doi.org/10.3390/molecules27010099.
[14]. Goedert, M., 2004, Tau
protein and Neurodegeneration. Seminars in cell & developmental biology,
15(1), 45–9, http://dx.doi.org/10.1016/j.semcdb.2003.12.015.
[16]. Ashraf, G. M., and Alexiou, A., 2019, Biological,
Diagnostic and Therapeutic Advances in Alzheimer's Disease. Springer
Nature,
293 p, https://play.google.com/store/books/details?id=N8m1DwAAQBAJ.
[17]. Hirshasri, A.G., Renu, K., Priya, V.V., Gayathri, R. and
Kavitha, S., The Effect of Aspalathin on SMAD2, SMAD3, TGF-β-A Major
Contributor of Inflammation–An In-silico Approach.
[18]. Roy, A., Cheriyan, B.V., Perumal, E., Rengasamy, K.R. and
Anandakumar, S., 2024. Effect of hinokitiol in ameliorating oral cancer: in
vitro and in silico evidences. Odontology, pp.1-14.
[19]. Saini, R.S., Binduhayyim, R.I.H., Gurumurthy, V., Alshadidi,
A.A.F., Aldosari, L.I.N., Okshah, A., Kuruniyan, M.S., Dermawan, D., Avetisyan,
A., Mosaddad, S.A. and Heboyan, A., 2024. Dental biomaterials redefined:
molecular docking and dynamics-driven dental resin composite
optimization. BMC Oral Health, 24(1), p.557.
[20]. Priya, V., Keerthivasan, S. and Kaviyarasi, R., Determining
the Dual Effect of Mirabegron on Anticancer Mechanism and Brown Adipose Tissue
Activation-An in-silico Approach.
[21]. Neelam, S., Gokara, M., Sudhamalla, B.,
Amooru, D. G. and Subramanyam, R., 2010, Interaction Studies of Coumaroyltyramine
with Human Serum Albumin and its Biological Importance. The Journal of
Physical Chemistry B, 114(8), pp.3005-3012, https://pubs.acs.org/doi/abs/10.1021/jp910156k.
[22]. Saini, R.S., Vaddamanu, S.K., Dermawan, D., Bavabeedu, S.S.,
Khudaverdyan, M., Mosaddad, S.A. and Heboyan, A., 2024. In Silico Docking of
Medicinal Herbs Against P. gingivalis for Chronic Periodontitis
Intervention. International Dental Journal.
[23]. Alfadda, A. A., Benabdelkamel, H., Masood,
A., Jammah, A. A. and Ekhzaimy, A. A., 2018, Differences in the plasma proteome
of patients with hypothyroidism before and after thyroid hormone replacement: A
proteomic analysis. International Journal of Molecular Sciences, 19(1),
p.88, https://www.mdpi.com/1422-0067/19/1/88.
[24]. Yang, J., Zhi, W., and Wang, L., 2024,
Role of Tau Protein in Neurodegenerative Diseases and Development of Its
Targeted Drugs: A Literature Review. Molecules, 29(12), p.2812, https://www.mdpi.com/1420-3049/29/12/2812.

