Asarone Possesses Antiproliferative Potential in Breast Cancer Cell Line (MCF-7) Through Via Apoptosis and Inflammatory-Mediated Signaling Pathways
Abstract:
Breast cancer is a significant global health challenge,
requiring continuous exploration of new treatments. Asarone, a bioactive
compound from the Acorus genus, shows promising anticancer properties but its
effects on breast cancer cells are underexplored. This study investigates
asarone's anticancer potential against breast cancer cell lines using in vitro
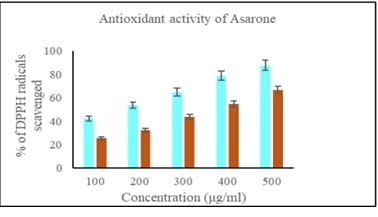
and in silico approaches. Asarone's antioxidant activity was evaluated using
DPPH radical scavenging assays, revealing a dose-dependent (25.56, 32.18, 47.73,
54.83 and 66.74%) effect on free radicals. MTT assays showed a dose-dependent
decrease in cell viability, indicating asarone's cytotoxicity towards breast
cancer cells. mRNA expression analysis showed that targeting apoptosis
regulators such as Bax (1, 1.3, 1.52 fold change upregualtion) and Bad (1, 1.4,
and 1.6 fold upregulation) gene expression demonstrated that asarone induces
apoptosis via the intrinsic pathway. Additionally, asarone inhibited Akt mNRA
(1, 0.6, and 0.4 fold change down regulation), caspase-3 (1, 1.4, and 1.7
upregulation) and cytochrome-c mRNA (1, 1.2 and 1,54 fold change upregulation) suggesting
interference with key cancer progression pathways. Molecular docking studies
predicted favorable binding interactions between asarone and crucial proteins
involved in apoptosis and cell survival, including Bax, Bad, cytochrome c,
caspase 3, and Akt. These findings collectively highlight the multifaceted
anticancer mechanisms of asarone against breast cancer cells. This study
underscores the potential of asarone as a natural therapeutic agent for breast
cancer, offering avenues for further exploration in translational research and
clinical trials. The current study significantly advances our understanding of
asarone's anticancer properties, offering promising directions for developing
new and effective breast cancer therapies.
References:
[1]. Alkabban, F. M., Ferguson, T., 2022, Breast Cancer. In: StatPearls.
Treasure Island (FL): StatPearls Publishing; September 26.
[2]. Jayaraman, S., Raj Natarajan, S., Ponnusamy, B., Veeraraghavan, V.P., Jasmine, S.,
2023, Unlocking the potential
of beta sitosterol: Augmenting the suppression of oral cancer cells through
extrinsic and intrinsic signalling mechanisms. Saudi Dent J,35(8):1007-1013. doi: 10.1016/j.sdentj.2023.08.003.
[3]. Janani, K. S., Gayatri Devi, R., Selvaraj, J., 2022, Antiproliferative effect of Merremia emarginata (Burm. F.)
leaf extract on SAOS-2 cell line. J Pharm Negat Results, 13:1805-1810. doi:10.47750/pnr.2022.13.S06237.
[4]. JinJin, P., Yuqiang, Y., Selvaraj, J., Ponnulakshmi, R., Prabhu Manickam, N,, Vidhya Rekha, U, Sridevi, G., Jeane Rebecca, R., Janaki, C. S., Dwarakesh, T., Chella Perumal., Monica, M., 2024, A review on advancements in the application of
starch-based nanomaterials in biomedicine: Precision drug delivery and cancer
therapy. International
Journal of Biological Macromolecules, 26(1):130746.,https://doi.org/10.1016/j.ijbiomac.2024.130746.
[5]. Benedict, A., Suresh, V., Muthamizh, S., Jayaraman, S., &
Hussein, M. A., 2024, Merremia emarginata Extract Potentiating the Inhibition
of Human Colon Cancer Cells (HT-29) via the Modulation of Caspase-3/Bcl-2
Mediated Pathways. Curēus, https://doi.org/10.7759/cureus.56300.
[6]. Roy,
J. R., Janaki, C. S., Jayaraman, S., Veeraraghavan, V. P., Periyasamy, V.,
Balaji, T., Vijayamalathi, M., Bhuvaneswari, P., Swetha, P., 2023, Hypoglycemic
Potential of Carica Papaya in Liver Is Mediated through
IRS-2/PI3K/SREBP-1c/GLUT2 Signaling in High-Fat-Diet-Induced Type-2 Diabetic Male Rats. Toxics,
11(3):240. doi: 10.3390/toxics11030240.
[7]. Butti, R., Das, S., Gunasekaran, V.P., Yadav, A.S., Kumar, D., Kundu, G.C., 2018, Receptor tyrosine kinases (RTKs) in breast cancer:
signaling, therapeutic implications and challenges. Mol Cancer,17(1):34. doi:10.1186/s12943-018-0797-x.
[8]. Chellian, R., Pandy, V., Mohamed, Z., 2017, Pharmacology and toxicology of α- and β-Asarone:
A review of preclinical evidence. Phytomedicine, 32:41-58. doi:10.1016/j.phymed.2017.04.003
[9]. Hatano, T., Edamatsu, R., Hiramatsu, M., MORI, A., Fujita,
Y., Yasuhara, T., OKUDA, T., 1989, Effects of the interaction of tannins with
co-existing substances. VI.: effects of tannins and related polyphenols on
superoxide anion radical, and on 1, 1-Diphenyl-2-picrylhydrazyl radical. Chemical
and Pharmaceutical Bulletin, 37(8):2016-2021.
[10]. Perumal, S., Langeshwaran, K., Selvaraj,
J., Ponnulakshmi, R., Shyamaladevi, B., Balasubramanian, M. P., 2018, Effect of
diosmin on apoptotic signaling molecules in N-nitrosodiethylamine-induced
hepatocellular carcinoma in experimental rats. Molecular and Cellular
Biochemistry, 449: 27-37.
[11]. Zhang, X., Abdelrahman, A., Vollmar, B.,
& Zechner, D., 2018, The Ambivalent Function of YAP in Apoptosis and
Cancer. International journal of molecular sciences, 19(12), 3770.
https://doi.org/10.3390/ijms19123770.
[12]. Ramalingam, K., Yadalam, P. K., Ramani, P., Krishna, M.,
Hafedh, S., Badnjević, A., Cervino, G., & Minervini, G., 2024, Light
gradient boosting-based prediction of quality of life among oral cancer-treated
patients. BMC oral health, 24(1), 349. https://doi.org/10.1186/s12903-024-04050-x
[13]. Neralla, M., M, H., Preethi, A., Selvakumar, S. C., &
Sekar, D., 2024, Expression levels of microRNA-7110 in oral squamous cell
carcinoma. Minerva Dental and Oral Science, 73(3), 155–160. https://doi.org/10.23736/S2724-6329.23.04801-5
[14]. Prasad, M., Jayaraman, S., Rajagopal, P., Veeraraghavan, V. P., Kumar, P.
K., Piramanayagam, S., Pari, L.,
2022, Diosgenin inhibits ER
stress-induced inflammation in aorta via iRhom2/TACE mediated signaling in
experimental diabetic rats: An in vivo and in silico approach. Chem Biol
Interact,358:109885. doi: 10.1016/j.cbi.2022.109885.
[15].
Kumar Subramanian,
Aravind & Katyal, Deepika., 2023, The Effect of Topical Melatonin Gel on
the oral health and salivary nickel and chromium content of orthodontic Patients:
An In Vivo Study. World Journal of Dentistry. 14. 326-330.
10.5005/jp-journals-10015-2218.
[16].
Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy,
V., Balaji, T., Vijayamalathi, M., Veeraraghavan, V. P., 2022, Effect of Carica
papaya on IRS-1/Akt signaling mechanisms in
High-Fat-Diet–Streptozotocin-Induced type 2 diabetic experimental rats: a
mechanistic approach. Nutrients, 14(19):4181.
[17]. Selvaraj, J., Veeraraghavan, V., Periyasamy, V., and Rajagopal, P., 2021., In Silico
and in Vitro Study on the Inhibition
of FtsZ Protein of Staphylococcus Aureus
by Active Compounds from Andrographis
Paniculata. Journal of Biologically
Active Products from Nature, 11(2): 116–128.
doi:10.1080/22311866.2021.1908163.
[18]. Ponnulakshmi, R., Shyamaladevi, B., Vijayalakshmi, P., &
Selvaraj, J., 2019, In silico and in vivo analysis to identify the
antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and
sucrose induced type-2 diabetic experimental rats. Toxicology Mechanisms and Methods, 29(4): 276–290. https://doi.org/10.1080/15376516.2018.1545815.
[19]. Babu, S., Jayaraman, S.,
2020, An update on β-sitosterol: A
potential herbal nutraceutical for diabetic management. Biomed Pharmacother, 131:110702. doi:
10.1016/j.biopha.2020.110702. Epub 2020 Aug 31. PMID: 32882583.
[20]. Guo, J., Gan, C., Cheng, B., Cui, B., Yi, F., 2023, Exploration of binding mechanism of apigenin to pepsin: Spectroscopic analysis, molecular docking, enzyme activity and antioxidant assays. pectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 290:122281. doi:10.1016/j.saa.2022.122281.

