Molecular Mechanisms Underlying in the Anticancer Activity of Verbacoside Against Human Lung Adeno Carcinoma (A549) Cells Via Modulating Apoptotic Signalling
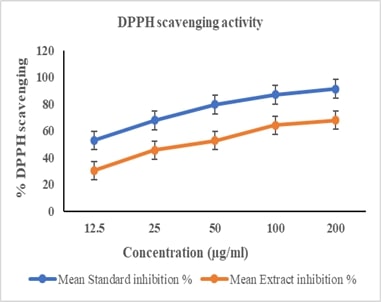
Abstract:
Verbascoside (VERB),
a phenylethanoid-phenylpropanoid glycoside, has garnered significant interest
due to its potential therapeutic effects, particularly its anticancer
properties. This study investigates the molecular mechanisms underlying the
anticancer activity of VERB against A549 cells, a model of non-small cell lung
cancer (NSCLC). Our findings demonstrate that VERB induces apoptosis in A549
cells through the modulation of key apoptotic signaling pathways. Specifically,
VERB treatment resulted in the activation of caspases, upregulation of
pro-apoptotic proteins, and downregulation of anti-apoptotic proteins.
Additionally, VERB was observed to inhibit the NF-kB pathway, thereby reducing
inflammation and promoting apoptotic cell death. These results suggest that
VERB exerts its anticancer effects by targeting multiple cellular pathways
involved in cell survival and apoptosis, providing a promising avenue for the
development of novel NSCLC therapies.
References:
[1]. Siegel, R. L., Miller, K. D., &
Jemal, A., 2018, Cancer statistics, 2018. CA: a cancer journal for clinicians,
68(1), 7-30.
[2]. Ames, B. N., & Gold, L. S., 1998,
The causes and prevention of cancer: the role of environment. Biotherapy, 11,
205-220.
[3]. Yeung, S. C., Habra, M. A., &
Thosani, S. N., 2011, Lung cancer-induced paraneoplastic syndromes. Current
opinion in pulmonary medicine, 17(4), 260–268. https://doi.org/10.1097/MCP.0b013e328347bdba.
[4]. Van Schil, P. E., Sihoe, A. D.,
& Travis, W. D., 2013, Pathologic classification of adenocarcinoma of lung.
Journal of Surgical Oncology, 108(5), 320–326. https://doi.org/10.1002/jso.23397.
[5]. Li, X., Wang, S., Zhu, R., Li, H.,
Han, Q., & Zhao, R. C. , 2016, Lung tumor exosomes induce a
pro-inflammatory phenotype in mesenchymal stem cells via NFκB-TLR signaling
pathway. Journal of Hematology & Oncology, 9, 42. https://doi.org/10.1186/s13045-016-0269-y.
[6]. Al-Harbi, N. O., Imam, F.,
Al-Harbi, M. M., Ansari, M. A., Zoheir, K. M., Korashy, H. M., Sayed-Ahmed, M.
M., Attia, S. M., Shabanah, O. A., & Ahmad, S. F., 2016, Dexamethasone
Attenuates LPS-induced Acute Lung Injury through Inhibition of NF-κB, COX-2,
and Pro-inflammatory Mediators. Immunological Investigations, 45(4),
349–369. https://doi.org/10.3109/08820139.2016.1157814.
[7]. Lu, W. J., Lin, K. H., Hsu, M. J.,
Chou, D. S., Hsiao, G., & Sheu, J. R., 2012, Suppression of NF-κB
signalling by andrographolide with a novel mechanism in human platelets:
regulatory roles of the p38 MAPK-hydroxyl radical-ERK2 cascade. Biochemical Pharmacology,
84(7), 914–924. https://doi.org/10.1016/j.bcp.2012.06.030
[8]. Desai, B. N., Myers, B. R., &
Schreiber, S. L., 2002, FKBP12-rapamycin-associated protein associates with
mitochondria and senses osmotic stress via mitochondrial dysfunction.
Proceedings of the National Academy of Sciences of the United States of
America, 99(7), 4319–4324. https://doi.org/10.1073/pnas.261702698.
[9]. Luo, C., Zhu, Y., Jiang, T., Lu,
X., Zhang, W., Jing, Q., Li, J., Pang, L., Chen, K., Qiu, F., Yu, X., Yang, J.,
& Huang, J., 2007, Matrine induced gastric cancer MKN45 cells apoptosis via
increasing pro-apoptotic molecules of Bcl-2 family. Toxicology, 229(3),
245–252. https://doi.org/10.1016/j.tox.2006.10.020.
[10]. Liang, C. Z., Zhang, J. K., Shi,
Z., Liu, B., Shen, C. Q., & Tao, H. M., 2012, Matrine induces
caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo
through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Cancer
Chemotherapy and Pharmacology, 69(2), 317–331. https://doi.org/10.1007/s00280-011-1699-4
[11]. Nussbaumer, S., Bonnabry, P.,
Veuthey, J. L., & Fleury-Souverain, S., 2011, Analysis of anticancer drugs:
a review. Talanta, 85(5), 2265–2289. https://doi.org/10.1016/j.talanta.2011.08.034
[12]. Rostamabadi, H., Falsafi, S. R.,
& Jafari, S. M., 2019, Nanoencapsulation of carotenoids within lipid-based
nanocarriers. Journal of Controlled Release: Official Journal of the
Controlled Release Society, 298, 38–67. https://doi.org/10.1016/j.jconrel.2019.02.005.
[13]. Gil, E. deS., Enache, T. A., &
Oliveira-Brett, A. M., 2013, Redox behaviour of verbascoside and rosmarinic
acid. Combinatorial Chemistry & High Throughput Screening, 16(2),
92–97.
[14]. Oyourou, J. N., Combrinck, S.,
Regnier, T., & Marston, A., 2013, Purification, Stability and Antifungal
Activity of Verbascoside from Lippia
javanica and Lantana camara Leaf Extracts. Industrial Crops and Products,
43, 820-826. https://doi.org/10.1016/j.indcrop.2012.08.028.
[15]. Fan, Y., Xu, C., Li, J., Zhang, L.,
Yang, L., Zhou, Z., Zhu, Y., & Zhao, D., 2018, Ionic liquid-based
microwave-assisted extraction of verbascoside from Rehmannia root.
Industrial Crops and Products, 124, 59-65.
[16]. Hatano, T., Edamatsu, R.,
Hiramatsu, M., Mori, A., Fujita, Y., Yasuhara, T., Yoshida, T., & Okuda, T.,
1989, Effects of the interaction of tannins with co-existing substances. VI.:
effects of tannins and related polyphenols on superoxide anion radical, and on
1, 1-Diphenyl-2-picrylhydrazyl radical. Chemical and Pharmaceutical Bulletin,
37(8), 2016-2021.
[17]. Padmanabhan, P., & Jangle, S.
N., 2012, Evaluation of in-vitro anti-inflammatory activity of herbal
preparation, a combination of four medicinal plants. International Journal of
Basic and Applied Medical Sciences, 2(1), 109-116.
[18]. Elias, G., & Rao, M. N., 1988, Inhibition
of albumin denaturation and antiinflammatory activity of dehydrozingerone and
its analogs. Indian Journal of Experimental Biology, 26(7), 540–542.
[19]. Vajrabhaya, L. O., &
Korsuwannawong, S., 2018, Cytotoxicity evaluation of a Thai herb using
tetrazolium (MTT) and sulforhodamine B (SRB) assays. Journal of Analytical
Science and Technology, 9(1), 1-6.
[20]. Schmittgen, T. D., & Livak, K.
J., 2008, Analyzing real-time PCR data by the comparative C(T) method. Nature
Protocols, 3(6), 1101–1108. https://doi.org/10.1038/nprot.2008.73.
[21]. Jayaraman, S., Natarajan, S. R.,
Ponnusamy, B., Veeraraghavan, V. P., & Jasmine, S., 2023, Unlocking the
potential of beta-sitosterol: Augmenting the suppression of oral cancer cells
through extrinsic and intrinsic signalling mechanisms. The Saudi Dental
Journal, 35(8), 1007-1013.
[22]. Jayaraman, S., Natarajan, S. R.,
Veeraraghavan, V. P., & Jasmine, S., 2023, Unveiling the anti-cancer
mechanisms of calotropin: Insights into cell growth inhibition, cell cycle
arrest, and metabolic regulation in human oral squamous carcinoma cells
(HSC-3). Journal of Oral Biology and Craniofacial Research, 13(6),
704-713.
[23]. Jayaraman, S., Natararaj, S.,
&Veeraraghavan, V. P., 2024, Hesperidin Inhibits Oral Cancer Cell Growth
via Apoptosis and Inflammatory Signaling-Mediated Mechanisms: Evidence From In
Vitro and In Silico Analyses. Cureus, 16(2).
[24]. Jayaraman, S., Veeraraghavan, V.
P., Natarajan, S. R., & Jasmine, S., 2024, Exploring the therapeutic
potential of curcumin in oral squamous cell carcinoma (HSC-3 cells): Molecular
insights into hypoxia-mediated angiogenesis. Pathology-Research and Practice,
254, 155130.

