Determining the Dual Effect of Mirabegron on Anticancer Mechanism and Brown Adipose Tissue Activation - An in-silico Approach
Abstract:
Mirabegron, a β3-adrenoceptor agonist first developed
for treating overactive bladder, has shown unexpected impacts on cancer and
metabolic processes. Initially targeting the bladder's detrusor muscle, new
research has revealed its potential in cancer therapy and brown adipose tissue
(BAT) activation. This work employs silico approaches to evaluate how
Mirabegron impacts critical cellular pathways such as AMPK, mTOR, and UCP,
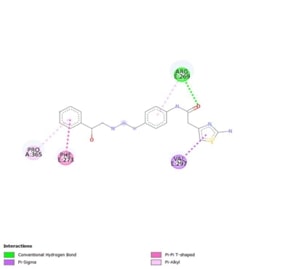
which are important for cancer and metabolic regulation. Docking studies show
that Mirabegron binds effectively to several targets, with high affinities
indicating a meaningful interaction. Specifically, it binds to AMPK at -7.0
kcal/mol, mTOR at -5.4 kcal/mol, and UCP at -7.4 kcal/mol. These interactions
contain key residues, indicating that Mirabegron's influence extends beyond its
original usage, potentially affecting cancer progression and metabolism.
References:
[1]. Bhide, A.A., Digesu, G.A., Fernando, R. and
Khullar, V., 2012. Mirabegron–a selective β3-adrenoreceptor agonist for the
treatment of overactive bladder. Research and Reports in Urology, 4,
p.41.https://doi.org/10.2147%2FRRU.S28930
[2]. Sun, K., Wang, D., Wu, G., Ma, J., Wang,
T., Wu, J. and Wang, J., 2021. Mirabegron improves the irritative symptoms
caused by BCG immunotherapy after transurethral resection of bladder tumors. Cancer
medicine, 10(21), pp.7534-7541.https://doi.org/10.1002/cam4.4278
[3]. Bel, J.S., Tai, T.C., Khaper, N. and Lees,
S.J., 2021. Mirabegron: The most promising adipose tissue beiging agent. Physiological
Reports, 9(5), p.e14779. https://doi.org/10.14814/phy2.14779
[4]. Kamal, M.M., El-Abhar, H.S., Abdallah,
D.M., Ahmed, K.A., Aly, N.E.S. and Rabie, M.A., 2024. Mirabegron, dependent on
β3-adrenergic receptor, alleviates mercuric chloride-induced kidney injury by
reversing the impact on the inflammatory network, M1/M2 macrophages, and
claudin-2. International Immunopharmacology, 126, p.111289.https://doi.org/10.1016/j.intimp.2023.111289
[5]. Chapple, C.R., Dvorak, V., Radziszewski,
P., Van Kerrebroeck, P., Wyndaele, J.J., Bosman, B., Boerrigter, P.,
Drogendijk, T., Ridder, A., Van Der Putten-Slob, I. and Yamaguchi, O., 2013. A
phase II dose-ranging study of mirabegron in patients with overactive bladder. International
Urogynecology Journal, 24, pp.1447-1458.https://doi.org/10.1007/s00192-013-2042-x
[6]. Bragg, R., Hebel, D., Vouri, S.M. and
Pitlick, J.M., 2014. Mirabegron: a beta-3 agonist for overactive bladder. The
Consultant Pharmacist®, 29(12), pp.823-837. DOI: https://doi.org/10.4140/TCP.n.2014.823
[7]. Jayaraman, S., Natararaj, S. and
Veeraraghavan, V.P., 2024. Hesperidin Inhibits Oral Cancer Cell Growth via
Apoptosis and Inflammatory Signaling-Mediated Mechanisms: Evidence From In
Vitro and In Silico Analyses. Cureus, 16(2).
https://doi.org/10.7759/cureus.53458
[8]. Sacco, E. and Bientinesi, R., 2012.
Mirabegron: a review of recent data and its prospects in the management of
overactive bladder. Therapeutic advances in Urology, 4(6), pp.315- 324.https://doi.org/10.1177/1756287212457114
[9]. Sun, X., Sui, W., Mu, Z., Xie, S., Deng,
J., Li, S., Seki, T., Wu, J., Jing, X., He, X. and Wang, Y., 2023. Mirabegron
displays anticancer effects by globally browning adipose tissues. Nature
Communications, 14(1), p.7610. https://doi.org/10.1038/s41467-023-43350-8.
[10]. Veeraraghavan, V.P., Jayaraman, S.,
Rengasamy, G., Mony, U., Ganapathy, D.M., Geetha, R.V. and Sekar, D., 2021.
Deciphering the role of microRNAs in neuroblastoma. Molecules, 27(1), p.99. https://doi.org/10.3390/molecules27010099
[11]. Calvani, M., Subbiani, A., Vignoli, M. and
Favre, C., 2019. Spotlight on ROS andβ3 Adrenoreceptors fighting in cancer
cells. Oxidative Medicine and Cellular Longevity, 2019(1), p.6346529. https://doi.org/10.1155/2019/6346529.
[12]. Kajimura, S., Seale, P., Kubota, K.,
Lunsford, E., Frangioni, J.V., Gygi, S.P. and Spiegelman, B.M., 2009.
Initiation of myoblast to brown fat switch by a PRDM16–C/EBP-β transcriptional
complex. Nature, 460(7259), pp.1154-1158.https://doi.org/10.1038/nature08262
[13]. Zhang, D., Sun, F., Yao, H., Bao, X., Wang,
D., Cui, Y. and Wu, J., 2021. The efficacy and safety of mirabegron for the
treatment of neurogenic lower urinary tract dysfunction: a systematic review
and meta-analysis. Frontiers in Pharmacology, 12, p.756582. https://doi.org/10.3389/fphar.2021.756582
[14]. Steinberg, J.D., Vogel, W. and Vegt, E.,
2017. Factors influencing brown fat activation in FDG PET/CT: a retrospective
analysis of 15,000+ cases. The British Journal of Radiology, 90(1075),
p.20170093. https://doi.org/10.1259/bjr.20170093.
[15]. Nedergaard, J. and Cannon, B., 2010. The
changed metabolic world with human brown adipose tissue: therapeutic visions.
Cell metabolism, 11(4), pp.268- 272. DOI: https://doi.org/10.1016/j.cmet.2010.03.007
[16]. Cannon, B. and Nedergaard, J.A.N., 2004.
Brown adipose tissue: function and physiological significance. Physiological
Reviews. https://doi.org/10.1152/physrev.00015.2003
[17]. Dawood O, El-Zawahry A. Mirabegron. In:
StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
[18]. Sui, W., Li, H., Yang, Y., Jing, X., Xue,
F., Cheng, J., Dong, M., Zhang, M., Pan, H., Chen, Y. and Zhang, Y., 2019.
Bladder drug mirabegron exacerbates atherosclerosis through activation of brown
fat-mediated lipolysis. Proceedings of the National Academy of Sciences,
116(22), pp.10937- 10942. https://doi.org/10.1073/pnas.1901655116.
[19]. Hardie, D.G., 2007. AMP-activated/SNF1
protein kinases: conserved guardians of cellular energy. Nature reviews
Molecular Cell Biology, 8(10), pp.774-785. https://doi.org/10.1038/nrm2249
[20]. Laplante, M. and Sabatini, D.M., 2012. mTOR
signaling in growth control and disease. cell, 149(2), pp.274-293. DOI: https://doi.org/10.1016/j.cell.2012.03.017
[21]. Chaudhary, R., Gupta, S. and Chauhan, S.,
2023. Protein uncoupling as an innovative practice in diabetes mellitus
treatment: A metabolic disorder. Endocrine, Metabolic & Immune
Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine &
Metabolic Disorders), 23(4), pp.494- 502. DOI: https://doi.org/10.2174/1871530322666220902143401
[22]. Ikeda, K., Kang, Q., Yoneshiro, T.,
Camporez, J.P., Maki, H., Homma, M., Shinoda, K., Chen, Y., Lu, X., Maretich,
P. and Tajima, K., 2017. UCP1-independent signaling involving SERCA2b-mediated
calcium cycling regulates beige fat thermogenesis and systemic glucose
homeostasis. Nature Medicine, 23(12), pp.1454-1465.https://doi.org/10.1038/nm.4429
[23]. Kannan, B. and Arumugam, P., 2023. The
implication of mitochondrial DNA mutation and dysfunction in periodontal
diseases. Journal of Indian Society of Periodontology, 27(2),
pp.126-130. doi: 10.4103/jisp.jisp_678_21.
[24]. Rieshy, V., Chokkattu, J.J., Rajeshkumar,
S. and Neeharika, S., 2023. Mechanism of action of clove and ginger herbal
formulation-mediated TiO2 nanoparticles against lactobacillus species: an in
vitro study. Journal of Advanced Oral Research, 14(1),
pp.61-66. https://doi.org/10.1177/23202068221142
[25]. Manivannan, H.P., Veeraraghavan, V.P. and
Francis, A.P., 2024. Identification of Novel Marine Bioactive Compound as
Potential Multiple Inhibitors in Triple-negative Breast Cancer-An in silico
Approach. Current Computer-aided Drug Design.
https://doi.org/10.2174/0115734099287118240102112337.
[26]. Hao, L., Scott, S., Abbasi, M., Zu, Y.,
Khan, M.S.H., Yang, Y., Wu, D., Zhao, L. and Wang, S., 2019. Beneficial
metabolic effects of mirabegron in vitro and in high-fat diet-induced obese
mice. Journal of Pharmacology and Experimental Therapeutics, 369(3),
pp.419-427. Doi: https://doi.org/10.1124/jpet.118.255778
[27]. Thomas, P., Selvakumar, S.C., Preethi,
K.A., Ramasubramanian, A., Ramani, P. and Sekar, D., 2023. miRNA-20a: A Dual
Regulator of Cell Migration and Apoptosis in Oral Squamous Cell Carcinoma:–An In
Vitro Study. Journal of Orofacial Sciences, 15(2), pp.167-174. https://ouci.dntb.gov.ua/en/works/4VOQ3DZl/
[28]. Thomas, P., Preethi, K.A., Selvakumar,
S.C., Ramani, P. and Sekar, D., 2023. Relevance of micro-RNAs and their targets
as a diagnostic and prognostic marker in oral squamous cell carcinoma. Journal
of Oral and Maxillofacial Pathology, 27(2), pp.364-373. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10581285/
[29]. Zhao, L., Luo, H., Li, T., Zhao, X. and
Liu, Y., 2022. β3 adrenoceptor agonist mirabegron protects against right
ventricular remodeling and drives Drp1 inhibition. Cardiovascular Diagnosis
and Therapy, 12(6), p.815. Doi: 10.21037/cdt-22-274
[30]. Dąbrowska, A.M. and Dudka, J., 2023.
Mirabegron, a selective β3-adrenergic receptor agonist, as a potential
anti-obesity drug. Journal of Clinical Medicine, 12(21), p.6897. https://doi.org/10.3390/jcm12216897
[31]. Anees, F.F., Preethi, K.A., Selvakumar,
S.C., Murthykumar, K., Ganapathy, D. and Sekar, D., 2023. Prospective study:
expression levels of microRNA-7-3p and its target STAT3
in head and neck cancer. Minerva Dental and Oral Science. https://pubmed.ncbi.nlm.nih.gov/37326506/.
[32]. Kaarthikeyan, G., Jayakumar, N.D. and
Sivakumar, D., 2019. Comparative Evaluation of Bone Formation between PRF and
Blood Clot Alone as the Sole Sinus-Filling Material in Maxillary Sinus
Augmentation with the Implant as a Tent Pole: A Randomized Split-Mouth
Study. Journal of long-term effects of medical implants, 29(2).
[33]. Kavarthapu, A. and Malaiappan, S., 2019.
Comparative evaluation of demineralized bone matrix and type II collagen
membrane versus eggshell powder as a graft material and membrane in rat
model. Indian Journal of Dental Research, 30(6),
pp.877-880.
[34]. Manchery, N., John, J., Nagappan, N., Subbiah, G.K. and Premnath, P., 2019. Remineralization potential of dentifrice containing nanohydroxyapatite on artificial carious lesions of enamel: A comparative: in vitro: study. Dental research journal, 16(5), pp.310-317.

