Curcumin Versus Curcumin-Iron Complex Docking in Tuberculosis Targets: A Insights on Synthesis & Molecular Docking Study
Abstract:
In this contemporary state Natural products play a
significant role in drug discovery. This research investigates a Curcumin-Iron
complex (CuFe) as a potential new weapon against tuberculosis (TB). The study highlights the global burden of TB
and the need for alternatives due to rising drug resistance. Curcumin, a
promising compound from turmeric, suffers from poor absorption in the body. The
researchers created CuFe and confirmed its structure using XRD analysis to
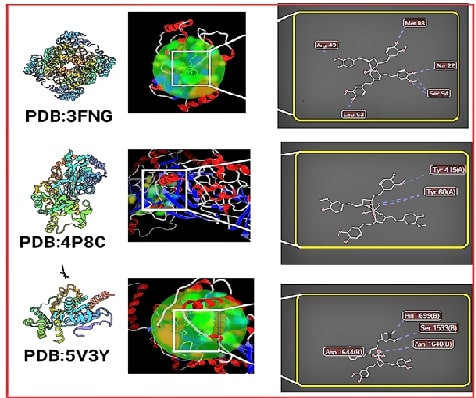
address this. Using computer simulations, they then tested CuFe's binding to
key targets in the TB bacteria. Excitingly, CuFe showed superior binding
compared to curcumin and existing drugs.
The discussion emphasizes the potential of plant-based medicines like
curcumin, along with metal complexes, mentioning garlic and piperine as further
avenues. The authors conclude that CuFe is a promising candidate, but further
testing in animals and humans, along with studies on absorption and regulatory
approval, is needed before it can be considered a viable TB treatment.
References:
[1].
Oryema, C., Rutaro, K., Oyet, S.W., and
Malinga, G.M., 2021. Ethnobotanical plants used in the management of symptoms
of tuberculosis in rural Uganda. Tropical Medicine and Health.; 49, 92.
10.1186/s41182-021-00384-2.
[2].
Mangwani, N., Singh, P.K., and Kumar, V.,
2020. Medicinal plants: Adjunct treatment to tuberculosis chemotherapy to
prevent hepatic damage. Journal of Ayurveda and integrative medicine.; 11,
522-528. 10.1016/j.jaim.2019.02.004.
[3].
Terreni, M., Taccani, M., and Pregnolato,
M.,2021. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest
Research Developments and Future Perspectives. Molecules (Basel, Switzerland).;
26. 10.3390/molecules26092671.
[4].
Sharifi-Rad, J., Rayess, Y.E., Rizk,
A.A., Sadaka, C., Zgheib, R., Zam, W., Sestito, S., Rapposelli, S.,
Neffe-Skocińska, K., Zielińska, D., et al., 2020. Turmeric and Its Major
Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food,
Pharmaceutical, Biotechnological and Medicinal Applications. Frontiers in
pharmacology.; 11, 01021. 10.3389/fphar.2020.01021.
[5].
Iweala, E.J., Uche, M.E., Dike, E.D.,
Etumnu, L.R., Dokunmu, T.M., Oluwapelumi, A.E., Okoro, B.C., Dania, O.E.,
Adebayo, A.H., and Ugbogu, E.A., 2023. Curcuma longa (Turmeric): Ethnomedicinal
uses, phytochemistry, pharmacological activities and toxicity profiles—A
review. Pharmacological Research - Modern Chinese Medicine .; 6, 100222.
https://doi.org/10.1016/j.prmcm.2023.100222.
[6].
Naksuriya, O., Okonogi, S., Schiffelers,
R.M., and Hennink, W.E., 2014. Curcumin nanoformulations: A review of
pharmaceutical properties and preclinical studies and clinical data related to
cancer treatment. Biomaterials.; 35, 3365-3383. https://doi.org/10.1016/j.biomaterials.2013.12.090.
[7].
Idoudi, S., Bedhiafi, T., Hijji, Y.M.,
and Billa, N., 2022. Curcumin and Derivatives in Nanoformulations with
Therapeutic Potential on Colorectal Cancer. AAPS PharmSciTech.; 23, 115.
10.1208/s12249-022-02268-y.
[8].
Iranshahi, M., Chini, M.G., Masullo, M.,
Sahebkar, A., Javidnia, A., Chitsazian Yazdi, M., Pergola, C., Koeberle, A.,
Werz, O., Pizza, C., et al., 2015. Can
Small Chemical Modifications of Natural Pan-inhibitors Modulate the Biological
Selectivity? The Case of Curcumin Prenylated Derivatives Acting as HDAC or
mPGES-1 Inhibitors. Journal of Natural Products 78, 2867-2879.
10.1021/acs.jnatprod.5b00700.
[9].
Delpu, Y., Cordelier, P., Cho, W.C., and
Torrisani, J., 2013. DNA Methylation and
Cancer Diagnosis. 14, 15029-15058.
[10]. Afzal, O., Yusuf, M., Ahsan, M.J., Altamimi, A.S.A.,
Bakht, M.A., Ali, A., and Salahuddin.,
2022. Chemical Modification of Curcumin into Its Semi-Synthetic Analogs
Bearing Pyrimidinone Moiety as Anticancer Agents. 11, 2737.
[11]. Venkatesan, P., and Rao, M.N.A., 2010.
Structure–Activity Relationships for the Inhibition of Lipid Peroxidation and
the Scavenging of Free Radicals by Synthetic Symmetrical Curcumin Analogues.
Journal of Pharmacy and Pharmacology 52, 1123-1128. 10.1211/0022357001774886 %J
Journal of Pharmacy and Pharmacology.
[12]. Hatamie, S., Nouri, M., Karandikar, S.K., Kulkarni,
A., Dhole, S.D., Phase, D.M., and Kale, S.N., 2012. Complexes of cobalt
nanoparticles and polyfunctional curcumin as antimicrobial agents. Materials
Science and Engineering: C 32, 92-97. https://doi.org/10.1016/j.msec.2011.10.002.
[13]. Refat, M.S., 2013. Synthesis and characterization of
ligational behavior of curcumin drug towards some transition metal ions:
Chelation effect on their thermal stability and biological activity.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 105,
326-337. https://doi.org/10.1016/j.saa.2012.12.041.
[14]. Sun, X., Tsang, C.-N., and Sun, H., 2008.
Identification and characterization of metallodrug binding proteins by
(metallo)proteomics†. Metallomics 1, 25-31. 10.1039/b813121j %J Metallomics.
[15]. Vaithiyalingam, M., Sumathi, D.L., and Sabarathinam,
S., 2023. Isolation and In silico Study of Curcumin from Curcuma longa and Its
Anti-Diabetic Activity. Applied biochemistry and biotechnology 195, 947-957.
10.1007/s12010-022-04173-3.
[16]. Bicer, N., Yildiz, E., Yegani, A.A., and Aksu, F.,
2018. Synthesis of curcumin complexes with iron(iii) and manganese(ii), and
effects of curcumin–iron(iii) on Alzheimer's disease. New Journal of Chemistry
42, 8098-8104. 10.1039/C7NJ04223J.
[17]. Vaithiyalingam, M., Mohan Kumar, R., Khagar, P.,
Sabarathinam, S., Alghazwani, Y., and Chidambaram, K., 2024. Isolation of
6-gingerol and semi-synthesis of 1,4-benzodiazepines derivatives: An in-situ
pharmacokinetics properties, molecular docking and molecular dynamics
simulation assessments. Saudi Journal of Biological Sciences 31, 104048.
10.1016/j.sjbs.2024.104048.
[18]. Sabarathinam, S., Ganamurali, N., Satheesh, S.,
Dhanasekaran, D., and Raja, A., 2023. Pharmacokinetic correlation of
structurally modified chalcone derivatives as promising leads to treat
tuberculosis. Future Medicinal Chemistry. 10.4155/fmc-2023-0161.
[19]. Yang, X., Liu, Y., Gan, J., Xiao, Z.-X., and Cao, Y.,
2022. FitDock: protein–ligand docking by template fitting. Briefings in
Bioinformatics 23. 10.1093/bib/bbac087.
[20]. Liu, Y., Grimm, M., Dai, W.T., Hou, M.C., Xiao, Z.X.,
and Cao, Y., 2020. CB-Dock: a web server for cavity detection-guided
protein-ligand blind docking. Acta pharmacologica Sinica 41, 138-144.
10.1038/s41401-019-0228-6.
[21]. Liu, Y., Yang, X., Gan, J., Chen, S., Xiao, Z.-X.,
and Cao, Y., 2022. CB-Dock2: improved protein–ligand blind docking by
integrating cavity detection, docking and homologous template fitting. Nucleic
Acids Research 50, W159-W164. 10.1093/nar/gkac394 %J Nucleic Acids Research.
[22]. Hartkoorn, R.C., Sala, C., Neres, J., Pojer, F.,
Magnet, S., Mukherjee, R., Uplekar, S., Boy-Röttger, S., Altmann, K.-H., and
Cole, S.T., 2012. Towards a new
tuberculosis drug: pyridomycin – nature's isoniazid. 4, 1032-1042.
https://doi.org/10.1002/emmm.201201689.
[23]. Rozwarski, D.A., Vilchèze, C., Sugantino, M.,
Bittman, R., and Sacchettini, J.C., 1999. Crystal Structure of the
Mycobacterium tuberculosis Enoyl-ACP Reductase, InhA, in Complex with NAD+ and
a C16 Fatty Acyl Substrate*. Journal of Biological Chemistry 274, 15582-15589.
https://doi.org/10.1074/jbc.274.22.15582.
[24]. Freundlich, J.S., Wang, F., Vilchèze, C., Gulten, G.,
Langley, R., Schiehser, G.A., Jacobus, D.P., Jacobs Jr., W.R., and Sacchettini,
J.C., 2009. Triclosan Derivatives: Towards Potent Inhibitors of Drug-Sensitive
and Drug-Resistant Mycobacterium tuberculosis. 4, 241-248.
https://doi.org/10.1002/cmdc.200800261.
[25]. Neres, J., Hartkoorn, R.C., Chiarelli, L.R.,
Gadupudi, R., Pasca, M.R., Mori, G., Venturelli, A., Savina, S., Makarov, V.,
Kolly, G.S., et al., 2015. 2-Carboxyquinoxalines Kill Mycobacterium
tuberculosis through Noncovalent Inhibition of DprE1. ACS Chemical Biology 10,
705-714. 10.1021/cb5007163.
[26]. Aggarwal, A., Parai, M.K., Shetty, N., Wallis, D.,
Woolhiser, L., Hastings, C., Dutta, N.K., Galaviz, S., Dhakal, R.C., Shrestha,
R., et al., 2017. Development of a Novel Lead that Targets M. tuberculosis
Polyketide Synthase 13. Cell 170, 249-259.e225. https://doi.org/10.1016/j.cell.2017.06.025.
[27]. Nikiforov, P.O., Surade, S., Blaszczyk, M., Delorme,
V., Brodin, P., Baulard, A.R., Blundell, T.L., and Abell, C., 2016. A fragment
merging approach towards the development of small molecule inhibitors of
Mycobacterium tuberculosis EthR for use as ethionamide boosters. Organic &
Biomolecular Chemistry 14, 2318-2326. 10.1039/C5OB02630J.
[28]. Willand, N., Dirié, B., Carette, X., Bifani, P.,
Singhal, A., Desroses, M., Leroux, F., Willery, E., Mathys, V., Déprez-Poulain,
R., et al., 2009. Synthetic EthR inhibitors boost antituberculous activity of
ethionamide. Nature Medicine 15, 537-544. 10.1038/nm.1950.
[29]. Zhang, Z., Bulloch, E.M., Bunker, R.D., Baker, E.N.,
and Squire, C.J. 2009., Structure and function of GlmU from Mycobacterium
tuberculosis. Acta crystallographica. Section D, Biological crystallography 65,
275-283. 10.1107/s0907444909001036.
[30]. Kumar, M., Singh, S.K., Singh, P.P., Singh, V.K.,
Rai, A.C., Srivastava, A.K., Shukla, L., Kesawat, M.S., Kumar Jaiswal, A.,
Chung, S.M., and Kumar, A., 2021. Potential Anti-Mycobacterium tuberculosis
Activity of Plant Secondary Metabolites: Insight with Molecular Docking
Interactions. Antioxidants (Basel, Switzerland) 10. 10.3390/antiox10121990.
[31]. Scarim, C.B., Lira de Farias, R., Vieira de Godoy
Netto, A., Chin, C.M., Leandro dos Santos, J., and Pavan, F.R., 202. Recent
advances in drug discovery against Mycobacterium tuberculosis: Metal-based
complexes. European Journal of Medicinal Chemistry 214, 113166.
https://doi.org/10.1016/j.ejmech.2021.113166.
[32]. Mjos, K.D., and Orvig, C., 2014. Metallodrugs in
Medicinal Inorganic Chemistry. Chemical Reviews .; 114, 4540-4563.
10.1021/cr400460s.
[33]. Pawar, A., Jha, P., Chopra, M., Chaudhry, U., and
Saluja, D., 2020. Screening of natural compounds that targets glutamate
racemase of Mycobacterium tuberculosis reveals the anti-tubercular potential of
flavonoids. Scientific Reports.; 10,
949. 10.1038/s41598-020-57658-8.
[34]. Sarangi, A., Das, B.S., Patnaik, G., Sarkar, S.,
Debnath, M., Mohan, M., and Bhattacharya, D.,2021. Potent anti-mycobacterial
and immunomodulatory activity of some bioactive molecules of Indian
ethnomedicinal plants that have the potential to enter in TB management.
Journal of applied microbiology., 131, 1578-1599. 10.1111/jam.15088.
[35]. Irfan, M., Lee, Y. Y., Lee, K. J., Kim, S. D., &
Rhee, M. H., 2022. Comparative antiplatelet and antithrombotic effects of red
ginseng and fermented red ginseng extracts. Journal of Ginseng Research, 46(3),
387-395.
[36]. Singla, N., Gupta, G., Kulshrestha, R., Sharma, K.,
Bhat, A. A., Mishra, R., Gupta, S., 2024. Daidzein in Traditional Chinese
Medicine: A Deep Dive into Its Ethnomedicinal and Therapeutic Applications.
Pharmacological Research-Modern Chinese Medicine, 12 (01) 100460.
[37]. Wang, J., Behl, T., Rana, T., Sehgal, A., Wal, P.,
Saxena, B., ... & Singla, R. K., 2024. Exploring the Pathophysiological
Influence of Heme Oxygenase-1 on Neuroinflammation and Depression: A Study of
Phytotherapeutic-Based Modulation. Phytomedicine,; 127 (07)155466.

