In-Silico Molecular Interaction and Pharmacokinetic Evaluation of Remimazolam and Major Intravenous Anesthetics Targeting GABAA Receptors
Abstract:
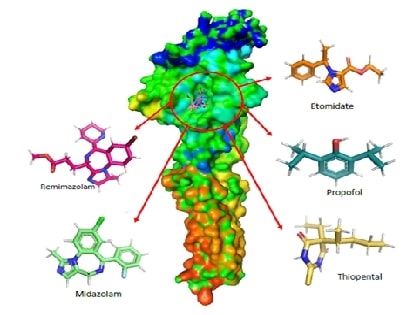
This study investigates the
molecular interactions and pharmacokinetic properties of five intravenous
anaesthetics Remimazolam, Midazolam, Propofol, Thiopental, and Etomidate with
the γ-Aminobutyric acid type A (GABAA) receptor, a key mediator of
inhibitory neurotransmission in the central nervous system. Using molecular
docking analysis, we evaluated the binding affinities of these drugs to the
GABAA neurotransmitter receptor. Remimazolam emerged as a promising
candidate with a docking score of -6.9 kcal/mol, demonstrating strong and
stable interactions with critical receptor residues such as THR96 and GLN65.
Although Midazolam exhibited a slightly superior docking score of -7.1
kcal/mol, Remimazolam’s pharmacokinetic profile offers distinct advantages,
including rapid onset, short duration of action, and a favourable safety
profile with minimal risk of hepatotoxicity and skin sensitization. In
comparison, Propofol, Thiopental, and Etomidate showed weaker binding
affinities and raised safety concerns. These findings suggest that Remimazolam
is a competitive and safer alternative to existing intravenous anaesthetics,
particularly in outpatient settings and procedures requiring efficient
anaesthetic management. This study contributes valuable insights into the
clinical application of Remimazolam, reinforcing its potential as an effective
choice in the realm of intravenous anaesthesia.
References:
[1].
Weir,
C. J., Mitchell, S. J., Lambert, J. J., 2017, Role of GABAA receptor subtypes
in the behavioural effects of intravenous general anaesthetics. Br J
Anaesth. 119: i167–i175. Doi:10.1093/bja/aex369.
[2].
Ramasamy,
K., Shanmugasundaram, J., Manoharan, R., Subramanian, V., Kathirvelu, P.,
Vijayaraghavan, R., 2022, Anti-neuropathic effect of 7,3′-dihydroxyflavone in
paclitaxel induced peripheral neuropathy in mice involving GABAA, KATP channel
and adenosine receptors. Neurochem Int. 159: 105388. Doi: 10.1016/j.neuint.2022.105388.
[3].
Marimuthu
M., 2021, Dental impactions performed under general anaesthesia - A retrospective
study on the frequency and implications, Int J Dent Oral Sci. 1793–1796.
Doi:10.19070/2377-8075-21000355.
[4].
Keerthika
S, Mani G., 2021, Knowledge, attitude and practice of dentists towards dental
procedures under general Anesthesia in children. J Pharm Res Int. 83–93.
Doi:10.9734/jpri/2021/v33i20B31361.
[5].
Manivasagam
D, Muthukrishnan A, Chaudary M., 2020, Assessment of effectiveness of local
anesthesia with and without adrenaline in patients with cardiac disorders. Int
J Pharm Res. 13. Doi:10.31838/ijpr/2021.13.01.217.
[6].
Dessai
S, Ninave S, Bele A., 2024, The Rise of remimazolam: A Review of pharmacology, clinical
efficacy, and safety profiles. Cureus. 16: e57260. Doi:10.7759/cureus.57260.
[7].
Kilpatrick
G. J., 2021, Remimazolam: Non-Clinical and clinical profile of a new
sedative/anesthetic agent. Front Pharmacol. 12: 690875. Doi:10.3389/fphar.2021.690875.
[8].
Noor
N, Legendre R, Cloutet A, Chitneni A, Varrassi G, Kaye A. D., 2021, A
comprehensive review of remimazolam for sedation. Heal Psychol Res.;9:
24514. Doi:10.52965/001c.24514.
[9].
Dao
V-A, Schippers F, Stöhr T., 2022, Efficacy of remimazolam versus midazolam for
procedural sedation: Post hoc integrated analyses of three phase 3 clinical
trials. Endosc Int open. 10: E378–E385. Doi:10.1055/a-1743-1936.
[10].
Dong
L, Sun T, Yang J, Zhou Y, Liu X, Liu Z, et al., 2024, Remimazolam has similar
anesthetic effect and superior safety compared to propofol in elderly patients:
A meta‐analysis of randomized controlled trials. World J Surg. Doi:10.1002/wjs.12273.
[11].
Hoshino
R, Ohashi N, Uta D, Ohashi M, Deguchi H, Baba H., 2024, Actions of remimazolam
on inhibitory transmission of rat spinal dorsal horn neurons. J Pharmacol
Sci. 155: 63–73. Doi: 10.1016/j.jphs.2024.04.002.
[12].
Masui
K., 2024, Remimazolam: Its clinical pharmacology and evolving role in
anesthesia and sedation practice. Curr Opin Anaesthesiol. 37: 344–351. Doi:10.1097/ACO.0000000000001384.
[13].
Kim,
K. M., 2022, Remimazolam: Pharmacological characteristics and clinical
applications in anesthesiology. Anesth pain Med. 17: 1–11. Doi:10.17085/apm.21115
[14].
Alharbi
K. S, Almalki W. H, Alzarea S. I, Kazmi I, Al-Abbasi F. A, Afzal O, et al.,
2024, Anaesthesia-induced changes in genomic expression leading to
neurodegeneration. CNS Neurol Disord - Drug Targets. 23: 411–419. Doi:10.2174/1871527322666230508123558.
[15].
Mahmoud
M, Mason KP., 2018, Recent advances in intravenous anesthesia and anesthetics.
F1000Research. 7: 470. Doi:10.12688/f1000research.13357.1
[16].
Berman
H. M, Battistuz T, Bhat T. N, Bluhm W. F, Bourne P. E, Burkhardt K, et al.,
2002, The protein data bank. Acta Crystallogr Sect D Biol Crystallogr.
58: 899–907. Doi:10.1107/S0907444902003451.
[17].
Miller
P. S, Aricescu A. R., 2014, Crystal structure of a human GABAA receptor. Nature.;512:
270–275. Doi:10.1038/nature13293.
[18].
Zielesny
A., 2005, Chemistry software package chemoffice ultra 2005. J Chem Inf
Model. 45: 1474–1477. Doi:10.1021/ci050273j.
[19].
Cousins
K., 1993, ChemOffice Plus: A package of programs for chemists. J Chem Inf
Comput Sci. 33: 788–789. Doi:10.1021/ci00015a603.
[20].
Morris
G. M, Huey R, Lindstrom W, Sanner M. F, Belew R. K, Goodsell D. S, et al.,
2010, AutoDock4 and AutoDockTools4: Automated docking with selective receptor
flexibility. J Comput Chem. 30: 2785–2791. Doi: 10.1002/jcc.21256.AutoDock4
[21].
The
PyMOL Molecular Graphics System, Version 2.0, Schrödinger, LLC. 2002.
Available: https://pymol.org
[22].
SYSTÈMES
D. BIOVIA Discovery Studio. Dassault Syst mes BIOVIA, Discovery Studio Modeling
Environment, Release 2017. Dassault Syst mes; 2016. Available: http://accelrys.com/products/collaborative-science/biovia-discovery-studio/
[23].
Pires
D. E V., Blundell T. L, Ascher D. B., 2015, pkCSM: Predicting small-molecule
pharmacokinetic and toxicity properties using graph-based signatures. J Med
Chem. 58: 4066–4072. Doi: 10.1021/acs.jmedchem.5b00104.
[24].
Jia
C-Y, Li J-Y, Hao G-F, Yang G-F., 2020, A drug-likeness toolbox facilitates
ADMET study in drug discovery. Drug Discov Today. 25: 248–258. Doi: 10.1016/j.drudis.2019.10.014.
[25].
Kesharwani
R. K, Vishwakarma V. K, Keservani R. K, Singh P, Katiyar N, Tripathi S., 2020,
Role of ADMET tools in current scenario: Application and limitations. Computer-Aided
Drug Design. Singapore: Springer Singapore. pp. 71–87. Doi:10.1007/978-981-15-6815-2_4.

