Policy Analysis of Regulatory Challenges for Medical Devices and In Vitro Diagnostics in Achieving Universal Health Coverage in Zimbabwe
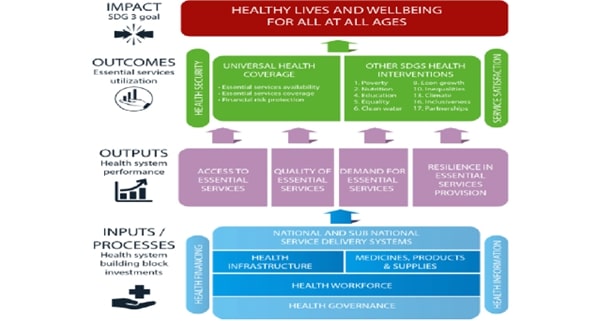
Abstract:
Zimbabwe's regulation of medical
devices and in vitro diagnostics (IVDs) lags behind that of medicines and
vaccines despite the country's goal of achieving Universal Health Coverage
(UHC) by 2030. This study, conducted from June to December 2022, rigorously
evaluated Zimbabwe's regulatory framework for medical devices and IVDs using a
comprehensive policy analysis framework. The study's methodology, which
included document review and comparative analysis, was designed to align the
regulatory framework with the UHC goal. The READ (Ready, Extract, Analyze, and
Distill) approach systematically assessed the relationship between medical
device regulations and national health strategic goals. Fourteen documents were
analysed, revealing that current regulations under the Medicines and Allied
Substances Control Act are insufficient for ensuring quality-assured medical
devices and IVDs due to a lack of explicit definitions and standards, leading
to inconsistent regulatory practices. The study found fragmented regulatory
approaches, overlapping institutional responsibilities, and a lack of effective
incorporation of medical device and IVD regulations in national health strategy
and related policies, hindering UHC achievement. The study recommends
comprehensive policy changes to harmonise regulations, clarify institutional
roles, and integrate medical device and IVD regulations into the national
health strategy. This ensures access to safe, effective, quality medical devices
and IVDs, promoting Zimbabwe's UHC goals by 2030.
References:
[1].
European Commission, 2017, MDR - Regulation
(EU) 2017/746 on in-vitro diagnostic medical devices: Official Journal of
the European Union, 1–175, https://www.emergogroup.com/sites/default/files/europe-medical-devices-regulation.pdf%0Ah
[2].
European Commission, 2017, Regulation
(EU) 2017/745 of The European Parliament and of the Council on Medical Devices,
Official Journal of the European Union, 5(8), 1-175, doi:
10.1177/2165079915576935.
[3].
Global Harmonization Task Force, 2006, Principles
of Medical Devices Classification, 1–27, https://www.imdrf.org/sites/default/files/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n15-2006-guidance-classification-060627.pdf
[4].
International Medical Devices Regulators
Forum, 2018, Essential Principles of Safety and Performance of Medical Devices,
International Medical Device Regulators Forum., 12-27, https://www.imdrf.org/sites/default/files/docs/imdrf/final/technical/imdrf-tech-181031-grrp-essential-principles-n47.pdf
[5].
World Health Organization, 2018, Health
products policy and standards, Good Manufacturing Practices https://www.who.int/teams/health-product-policy-and-standards/assistive-and-medical-technology/medical-devices/regulations
[6].
Hubner, S., Maloney, C., Phillips, S.
D., Doshi, P., Mugaga, J., Ssekitoleko, R. T, Mueller, J. L, Fitzgerald, T. N.,
2021, The evolving landscape of medical device regulation in East, Central, and
Southern Africa, Global Health Science and Practice, 9(1),
136–148, doi: 10.9745/GHSP-D-20-00578.
[7].
Dacombe, R., Watson,
V., Nyirenda, L., Madanhire, C., Simwinga, M., Chepuka, L., Johnson, C., Corbett,
E., Hatzold, K., Taegtmeyer, M., 2019, Regulation
of HIV self-testing in Malawi, Zambia and Zimbabwe: a qualitative study with
key stakeholders, Journal of the International AIDS Society, 22(S1), 5–11, doi:
10.1002/jia2.25229.
[8].
Reddy, K. S. and Mathur, M. R., 2018, Universal
Health Coverage, in Equity and Access, Oxford University Press, 305–322, doi:
10.1093/oso/9780199482160.003.0015.
[9].
World Health Organization, 2017, Leave
no one behind. Strengthening Health Systems for UHC and the SDGs in Africa, WHO
Regional Office for Africa, Licence: CC BY-NC-SA 3.0 IGO., 252–256, doi:
10.1080/15027570410006228.
[10]. World Health Organization, 2017, WHO global model regulatory
framework for medical devices including in vitro diagnostic medical devices,
WHO Medical Device Technical series. Licence: CC BY-NC-SA 3.0 IGO, https://www.who.int/medical_devices/publications/global_model_regulatory_framework_meddev/en/
[11]. Walt, G. and Gilson, L., 1994, Reforming the health sector in
developing countries: The central role of policy analysis, Health Policy and
Planning. Oxford Academic, 353–370, doi: 10.1093/heapol/9.4.353.
[12]. Kayesa, N. K. and Shung-King, M., 2021, The role of document
analysis in health policy analysis studies in low and middle-income countries:
Lessons for HPA researchers from a qualitative systematic review, Health
Policy OPEN, (2), 100024, doi: 10.1016/j.hpopen.2020.100024.
[13]. Walt, G., Schiffman, J., Schneider, H., Murray, S. F., Brugha, R., and Gilson, L., 2008, 'Doing' health policy
analysis: Methodological and conceptual reflections and challenges, Health
Policy and Planning. Oxford University Press, 308–317, doi:
10.1093/heapol/czn024.
[14]. Sidibé, C. S., Becquet, V., Brückner,
T. Y., Touré, O., Traoré, L. F., Broerse, J. E. W., and Dieleman, M., 2022, Adoption of harmonisation policy for the midwives’
training programme in Mali: A policy analysis, PLOS Global Public Health,
2(11), e0001296, doi: 10.1371/journal.pgph.0001296.
[15]. Walt, G. and Gilson, L., 1994, Reforming the health sector in
developing countries: The central role of policy analysis, Health Policy and
Planning. Oxford Academic, 353–370, doi: 10.1093/heapol/9.4.353.
[16]. Bowen, G. A., 2009, Document analysis as a qualitative
research method, Qualitative Research Journal, 9(2), 27–40, doi:
10.3316/QRJ0902027.
[17]. Dalglish, S. L., Khalid, H. and McMahon, S. A., 2020, Document
analysis in health policy research: The READ approach, Health Policy and
Planning, 35(10), 1424–1431, doi: 10.1093/heapol/czaa064.
[18]. World Health Organization (WHO), 2020, Guidance for
post-market surveillance and market surveillance of medical devices, including
in vitro diagnostics, 50, https://www.who.int/publications/i/item/9789240015319
[19]. De Maria, C., Di Pietro, L., Díaz
Lantada, A., Makobore, P. N., Mridha,
M., Ravizza, A., Torop, J., and Ahluwalia, A., 2018, Safe innovation: On medical device legislation in Europe and Africa,
Health Policy and Technology, 7(2), 156–165, doi:
10.1016/j.hlpt.2018.01.012.
[20]. World Health Organization (WHO), 2017, WHO Global Model
Regulatory Framework for Medical Devices including in vitro diagnostic medical
devices., WHO Medical Device Technical series. Licence: CC BY-NC-SA 3.0 IGO. http://apps.who.int/bookorders.%0Ahttp://apps.who.int/bookorders.%0Ahttps://www.who.int/medical_devices/publications/global_model_regulatory_framework_meddev/en/%0Ahttp://www.who.int/medicines/areas/quality_safety/quality_assurance/2016-07-27Modelregulato
[21]. Global Harmonization Task Force, 2012, Principles of
Conformity Assessment for Medical Devices, Ghtf/Sg1/N78:2012, https://www.imdrf.org/sites/default/files/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n78-2012-conformity-assessment-medical-devices-121102.pdf
[22]. Kuchenmüller, T., Chapman, E., Takahashi, R., Lester, L., Reinap,
M., Ellen, Haby, M. M., 2022, A comprehensive monitoring and evaluation
framework for evidence to policy networks, Evaluation and Program Planning,
91(102053), 1–23, doi: 10.1016/j.evalprogplan.2022.102053.A.
[23]. Allen, L. N., 2022, The philosophical foundations of “health for all” and Universal Health Coverage, International Journal for Equity in Health, 21(1), 1–7, doi: 10.1186/s12939-022-01780-8

