Studies on the Antioxidant Role of Β-Sitosterol on Neuronal Cell Line (IMR32) In Vitro: Role of NRF2-Keap Pathway
DOI: 10.21522/TIJPH.2013.SE.24.03.Art006
Authors : Selvaraj Jayaraman, Vishnu Priya Veeraraghavan, Ponnulakshmi Rajagopal, Chella Perumal Palanisamy, Ramajayam Govindan, S. Prathiba, R. Jayasree, Mahesh Kumar
Abstract:
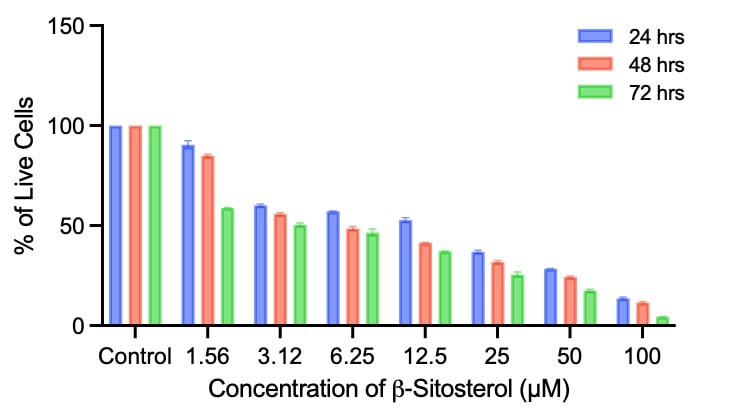
β-Sitosterol is a phytosterol which is universally present in most of the plant seeds and nuts. Its anti-inflammatory property has been studied in vitro in cell line studies and some in vivo studies have also been conducted but few studies have been done to establish its antioxidant property. In this study, we have attempted to explore the antioxidant property through the NRF2-KEAP pathway in the IMR 32 neuronal cell line. Initially, cell viability was checked for varying concentrations of sitosterol using an MTT assay. There was a decline in viability for increased concentration and time factors. Reactive oxygen species (ROS) assay revealed the tendency of sitosterol to inhibit lipid peroxides and hydrogen peroxides. An antioxidant activity assay depicted activating antioxidant enzymes, superoxide dismutase, and nonenzymatic antioxidants, such as reduced glutathione. In the gene expression analysis using Real Time-PCR NRF2 and KEAP expression was found to be observed maximum for concentration ranges of 10 µM concentration of β-Sitosterol. Current studies have substantiated the antioxidant property of β-Sitosterol through the NRF2- KEAP signalling pathway.References:
[1]. Zhang, P., Liu, N., Xue, M., Zhang, M., Liu, W., Xu, C., Fan, Y., Meng, Y., Zhang, Q., & Zhou, Y., 2023, Anti-Inflammatory and Antioxidant Properties of β-Sitosterol in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio). Antioxidants (Basel, Switzerland), 12(2), 391. https://doi.org/10.3390/antiox12020391
[2]. Sun, Y., Gao, L., Hou, W., & Wu, J., 2020, β-Sitosterol Alleviates Inflammatory Response via Inhibiting the Activation of ERK/p38 and NF-κB Pathways in LPS-Exposed BV2 Cells. BioMed Research International, 2020, 7532306. https://doi.org/10.1155/2020/7532306
[3]. Kaplanski, G., 2018, Interleukin-18: Biological Properties and Role in Disease Pathogenesis. Immunological Reviews, 281(1), 138–153. https://doi.org/10.1111/imr.12616
[4]. Prathipa, S., Shanmuga Sundaram, K. S., Geetha Rani., Krithika Chandrasekar., Ramajayam Govindan., Mahesh Kumar, P., Jaideep Mahendra., Ponnulakshmi,R., 2023, Phytosterols and its Neuroprotective Effect – An Updated Review, European Chemical Bulletin, J. Doi:10.48047/ecb/2023.12.si4.701
[5]. Zheng, Y., Zhao, J., Chang, S., Zhuang, Z., Waimei, S., Li, X., Chen, Z., Jing, B., Zhang, D., & Zhao, G., 2023, β-Sitosterol Alleviates Neuropathic Pain by Affect Microglia Polarization through Inhibiting TLR4/NF-κB Signaling Pathway. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology, 18(4), 690–703. https://doi.org/10.1007/s11481-023-10091-w
[6]. Baskar, A. A., Al Numair, K. S., Gabriel Paulraj, M., Alsaif, M. A., Muamar, M. A., & Ignacimuthu, S., 2012, β-sitosterol Prevents Lipid Peroxidation And Improves Antioxidant Status and Histoarchitecture in rats with 1, 2-Dimethylhydrazine-Induced Colon Cancer. Journal of Medicinal Food, 15(4), 335–343. https://doi.org/10.1089/jmf.2011.1780
[7]. Gupta, R., Sharma, A. K., Dobhal, M. P., Sharma, M. C., & Gupta, R. S., 2011, Antidiabetic and Antioxidant Potential of β-Sitosterol in Streptozotocin-Induced Experimental Hyperglycemia. Journal of Diabetes, 3(1), 29–37. https://doi.org/10.1111/j.1753-0407.2010.00107.x
[8]. Kaspar, J. W., Niture, S. K., & Jaiswal, A. K., 2009, Nrf2:INrf2 (Keap1) Signaling In Oxidative Stress. Free Radical Biology & Medicine, 47(9), 1304–1309. https://doi.org/10.1016/j.freeradbiomed.2009.07.035
[9]. Ye, J. Y., Li, L., Hao, Q. M., Qin, Y., & Ma, C. S., 2020, β-Sitosterol Treatment Attenuates Cognitive Deficits And Prevents Amyloid Plaque Deposition In Amyloid Protein Precursor/Presenilin 1 Mice. The Korean Journal Of Physiology & Pharmacology : official Journal of the Korean Physiological Society and The Korean Society of Pharmacology, 24(1), 39–46. https://doi.org/10.4196/kjpp.2020.24.1.39
[10]. Wang, J., Wu, F., & Shi, C., 2013, Substitution of Membrane Cholesterol with β-Sitosterol Promotes Nonamyloidogenic Cleavage of Endogenous Amyloid Precursor Protein. Neuroscience, 247, 227–233. https://doi.org/10.1016/j.neuroscience.2013.05.022
[11]. Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., & Ferri, C. P., 2013, The global prevalence of Dementia: A Systematic Review and Metaanalysis. Alzheimer's & Dementia: The Journal of the Alzheimer's Association, 9(1), 63–75.e2. https://doi.org/10.1016/j.jalz.2012.11.007
[12]. Tiwari, A., & Jain, R. K., 2023, Comparative Evaluation of White Spot Lesion incidence between NovaMin, Probiotic, And Fluoride containing Dentifrices during Orthodontic treatment Using Laser Fluorescence - A Prospective Randomized Controlled Clinical Trial. Clinical and Investigative Orthodontics, 82(2), 75–82. https://doi.org/10.1080/27705781.2023.2190950
[13]. Pratiwi, R., Nantasenamat, C., Ruankham, W., Suwanjang, W., Prachayasittikul, V., Prachayasittikul, S., & Phopin, K., 2021, Mechanisms and Neuroprotective Activities of Stigmasterol Against Oxidative Stress-Induced Neuronal Cell Death via Sirtuin Family. Frontiers in Nutrition, 8, 648995. https://doi.org/10.3389/fnut.2021.648995
[14]. Tomolonis, J. A., Xu, X., Dholakia, K. H., Zhang, C., Guo, L., Courtney, A. N., Wang, S., Balzeau, J., Barragán, G. A., Tian, G., Di Pierro, E. J., & Metelitsa, L. S., 2023, Interaction Between Tumor Cell TNFR2 and Monocyte Membrane-Bound TNF-α Triggers Tumorigenic Inflammation in Neuroblastoma. Journal for Immunotherapy of Cancer, 11(3), e005478. https://doi.org/10.1136/jitc-2022-005478
[15]. Sreenivasagan, S., Subramanian, A. K., Mohanraj, K. G., & Kumar, R. S., 2023, Assessment of Toxicity of Green Synthesized Silver Nanoparticle-coated Titanium Mini-implants with Uncoated Mini-implants: Comparison in an Animal Model Study. The Journal of Contemporary Dental Practice, 24(12), 944–950. https://doi.org/10.5005/jp-journals-10024-3577.
[16]. Shi, C., Wu, F., Zhu, X. C., & Xu, J., 2013, Incorporation of Beta-Sitosterol Into The Membrane Increases Resistance To Oxidative Stress And Lipid Peroxidation via Estrogen Receptor-Mediated PI3K/GSK3beta Signaling. Biochimica et Biophysica Acta, 1830(3), 2538–2544. https://doi.org/10.1016/j.bbagen.2012.12.012
[17]. Neralla, M., M, H., Preethi, A., Selvakumar, S. C., & Sekar, D., 2024, Expression levels of microRNA-7110 in oral squamous cell carcinoma. Minerva dental and oral science, 73(3), 155–160. https://doi.org/10.23736/S2724-6329.23.04801-5
[18]. Ayaz, M., Junaid, M., Ullah, F., Subhan, F., Sadiq, A., Ali, G., Ovais, M., Shahid, M., Ahmad, A., Wadood, A., El-Shazly, M., Ahmad, N., & Ahmad, S., 2017, Anti-Alzheimer's Studies on β-Sitosterol Isolated from Polygonum hydropiper L. Frontiers in Pharmacology, 8, 697. https://doi.org/10.3389/fphar.2017.00697
[19]. Babu, S., & Jayaraman, S., 2020, An update on β-sitosterol: A Potential Herbal Nutraceutical for Diabetic Management. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 131, 110702. https://doi.org/10.1016/j.biopha.2020.110702
[20]. Jayaraman, S., Devarajan, N., Rajagopal, P., Babu, S., Ganesan, S. K., Veeraraghavan, V. P., Palanisamy, C. P., Cui, B., Periyasamy, V., & Chandrasekar, K., 2021, β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules (Basel, Switzerland), 26(7), 2101. https://doi.org/10.3390/molecules26072101

