Molecular Docking Analysis of Epigallocatechin 3- Gallate [EGCG] on Fatty Acids and Carnitine Transporters Family
Abstract:
EGCG is the main catechin present in green tea. Fatty
acids can be categorized as saturated or unsaturated depending on the
hydrocarbon chain and terminal carboxyl group they contain, typically with an
even number of carbons. EGCG has attracted considerable interest because of its
several health benefits, such as its anti-inflammatory, antioxidant, and
anticancer characteristics. Yet, the precise molecular targets and mechanisms
of action are not fully understood. Exploring the potential interaction of EGCG
with fatty acids and carnitine transporters, which play a vital role in lipid
metabolism and energy production, could provide insights into its physiological
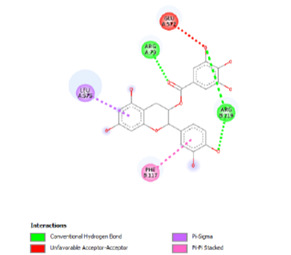
effects. Analysis of molecular docking between EGCG and fatty acid and
carnitine transporters, and their interactions. A contact and binding occur
between the fatty acid transporters (FABP) and VLCAD and CPTII. EGCG was
subjected to molecular docking simulations with active sites of transporter
families such as FABP, CPT2, and VLCAD. The docking analysis showed that EGCG
has favourable binding interactions with the target transporters, involving
important hydrogen bonding and hydrophobic interactions. EGCG showed
a strong ability to bind to the active sites of FABP and CPT2, indicating its
potential to influence their functions.
References:
[1] Nagle, D.G., Ferreira, D. and Zhou, Y.D., 2006. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry, 67(17), pp.1849-1855.https://pubmed.ncbi.nlm.nih.gov/16876833/.
[2] Mokra, D., Joskova, M. and Mokry, J., 2022. Therapeutic effects of green tea polyphenol (‒)-Epigallocatechin-3-Gallate (EGCG) in relation to molecular pathways controlling inflammation, oxidative stress, and apoptosis. International Journal of Molecular Sciences, 24(1), p.340.https://www.mdpi.com/1422-0067/24/1/340.
[3] Mokra, D., Adamcakova, J. and Mokry, J., 2022. Green tea polyphenol (-)-Epigallocatechin-3-Gallate (egcg): a time for a new player in the treatment of respiratory diseases?. Antioxidants, 11(8), p.1566.https://www.mdpi.com/2076-3921/11/8/1566.
[4] Bartlett, K. and Eaton, S., 2004. Mitochondrial β‐oxidation. European Journal of Biochemistry, 271(3), pp.462-469.https://febs.onlinelibrary.wiley.com/doi/full/10.1046/j.1432-1033.2003.03947.x
[5] Akar, H.T., Yıldız, Y., Mutluay, R., Tekin, E. and Tokatlı, A., 2023. Adult-onset carnitine palmitoyl transferase II (CPT II) deficiency presenting with rhabdomyolysis and acute kidney injury. CEN Case Reports, pp.1-5. https://link.springer.com/article/10.1007/s13730-023-00804-8.
[6] Palaniappan, C.S., Mohanraj, K.G. and Mathew, M.G., 2021. Knowledge And Awareness On The Association Between Physical Inactivity, Junk Food Consumption And Obesity Among Adolescent Population-A Survey Based Analysis. Int J Dentistry Oral Sci, 8(03), pp.1946-1951.https://paper.researchbib.com/view/paper/336893
[7] Daniel-E-mail, P., Vijayalakshmi–E-mail, T.M. and Krishnan-E-mail, M., 2023. Effect of lupeol on insulin resistance in adipose tissue by modulating the expression of insulin and inflammatory signaling molecules in high-fat diet and sucrose-fed diabetic rats. Bioinformation, 19(4), pp.445-453,https://www.bioinformation.net/019/97320630019445.pdf.
[8] Arora, D., Gayathri, R., Selvaraj, J., Vishnu Priya, V. and Kavitha, S., 2021. Vitamin C and E Down Regulates the Expression of C-JNK, IKKB, NF-kB in Adipose Tissue of PCB-Exposed Rats. Journal of Research in Medical and Dental Science, 9(11), pp.39-44, https://www.jrmds.in/articles/vitamin-c-and-e-down-regulates-the-expression-of-cjnk-ikkb-nfkb-in-adipose-tissue-of-pcbexposed-rats.pdf
[9] El-Ryalat, S.W., Irshaid, Y.M., Abujbara, M., El-Khateeb, M. and Ajlouni, K.M., 2023. Adipocyte “Fatty Acid Binding Protein” Gene Polymorphisms (and) in Jordanians with Obesity and Type 2 Diabetes Mellitus. Balkan Journal of Medical Genetics, 25(2), pp.63-70.https://europepmc.org/article/MED/37265971.
[10] Prasath, R. and Sinduja, P., 2023. Knowledge And Awareness on Various Treatment Modalities of Diabetes Mellitus-A Observational Survey. Journal for Educators, Teachers and Trainers, 13(6), 190-198, https://digibug.ugr.es/handle/10481/79933.
[11] Menon, G.R. and Sankari Malaiappan, K.K., 2021. Association Between Right Upper Molar Involvement And Diabetes Mellitus In Subjects With Chronic Periodon-titis. Int J Dentistry Oral Sci, 8(6), pp.2879-2884. https://paper.researchbib.com/view/paper/337088.
[12] Shah, P.M. and Chaudhary, M., 2020. Prevalence of diabetes mellitus among dental patients undergoing extractions-An institutional study. Journal of Complementary Medicine Research, 11(3), pp.278-278.https://www.semanticscholar.org/paper/Prevalence-of-diabetes-mellitus-among-dental-An-Shah-S./5a09ddcc56aecb40ba7ff3de3557f440189bf9f1
[13] Wan, C., Ouyang, J., Li, M., Rengasamy, K.R. and Liu, Z., 2022. Effects of green tea polyphenol extract and epigallocatechin-3-O-gallate on diabetes mellitus and diabetic complications: Recent advances. Critical Reviews in Food Science and Nutrition, pp.1-29.https://www.maxapress.com/article/doi/10.48130/BPR-2023-0032.
[14] Mathivadani, V., SMILINE GIRIJA, A.S. and Priyadharsini, J.V., 2020. Targeting Epstein-Barr virus nuclear antigen 1 (EBNA-1) with Murrayakoengii bio-compounds: An in-silico approach. Acta virologica, 64(1), 93–99,https://pubmed.ncbi.nlm.nih.gov/32180423/.
[15] McNutt, A.T., Francoeur, P., Aggarwal, R., Masuda, T., Meli, R., Ragoza, M., Sunseri, J. and Koes, D.R., 2021. GNINA 1.0: molecular docking with deep learning. Journal of cheminformatics, 13(1), pp.1-20.https://pubmed.ncbi.nlm.nih.gov/34108002/.
[16] Smiline Girija, A.S., 2020. Delineating the Immuno-Dominant Antigenic Vaccine Peptides Against gacS-Sensor Kinase in Acinetobacter baumannii: An in silico Investigational Approach. Frontiers in Microbiology, 11, p.2078, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7506167/.
[17] Kuppusamy, S.P., 2021. Lakshmi. T. Bioactive Compounds from Clove against Oral biofilm drug targets-An insilico Analysis. Int J Dentistry Oral Sci, 8(1), pp.1395-1398, http://dx.doi.org/10.19070/2377-8075-21000276.
[18] Prathap, L. and Jayaraman, S., 2022. Identification of Endogenous Superoxide Dismutase as a Potential Inhibitor for Pi3k/Akt Signaling In Colorectal Cancer-A Molecular Docking Study. Journal of Pharmaceutical Negative Results, pp.1374-1379,https://www.pnrjournal.com/index.php/home/article/view/1227.
[19] Padhi, S., Nayak, A.K. and Behera, A., 2020. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomedicine & Pharmacotherapy, 131, p.110708.https://pubmed.ncbi.nlm.nih.gov/32927252/.
[20] Lee, J., Hyon, J.Y., Min, J.Y., Huh, Y.H., Kim, H.J., Lee, H., Yun, S.H., Choi, C.W., Ha, S.J., Park, J. and Chung, Y.H., 2020. Mitochondrial carnitine palmitoyl transferase 2 is involved in Nε-(carboxymethyl)-lysine-mediated diabetic nephropathy. Pharmacological research, 152, p.104600.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10080702/.
[21] Xiang, J., Raka, R.N., Zhang, L., Xiao, J., Wu, H. and Ding, Z., 2022. Inhibition of three diabetes-related enzymes by procyanidins from Lotus (Nelumbo nucifera Gaertn.) seedpods. Plant Foods for Human Nutrition, 77(3), pp.390-398.https://pubmed.ncbi.nlm.nih.gov/35781857/.
[22] Ganesan A, Muthukrishnan A, Veeraraghavan V (2021) Effectiveness of Salivary Glucose in Diagnosing Gestational Diabetes Mellitus. Contemp Clin Dent 12:294–300
[23] Karthik EVG, Priya V (2021) Gayathri. R, Dhanraj Ganapathy. Health Benefits Of Annona Muricata-A Review. Int J Dentistry Oral Sci 8:2965–2967
[24] Priya DV, (2020) Knowledge and awareness on HIV/AIDS among college students in A university hospital setting. Int J Dent Oral Sci 1182–1186

