Fabrication, Characterisation, and Biocompatibility of Graphene-loaded Polymannose-Chitosan Scaffold for Potential Drug Screening Applications
Abstract:
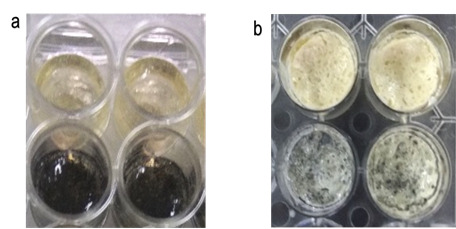
Graphene-loaded polymannose-chitosan scaffolds have emerged as promising candidates for drug screening applications due to their unique properties. The combination of polymannose and chitosan, supplemented with graphene, offers a versatile platform with potential applications including drug screening. The aim of this study is to fabricate and characterize the biocompatible graphene oxide loaded polymannose chitosan scaffold (PM-Chi-GO) for potential drug screening applications. The scaffold was meticulously prepared by combining oxidized polymannose with chitosan hydrochloride and graphene oxide, employing gelation techniques. Characterization involved Fourier Transform Infrared Spectroscopy (FTIR) for functional group analysis and Scanning Electron Microscopy (SEM) for morphological studies. The biocompatibility of the scaffold was assessed using Peripheral Blood Mononuclear Cells (PBMCs). FTIR analysis revealed distinctive peaks at 3296, 1624, 1528, 1389, 1060, and 808 cm-1, corresponding to specific functional groups within the scaffold. SEM displayed a porous morphological structure. Biocompatibility testing with PBMCs demonstrated favorable responses, confirming the scaffold's potential for in vitro drug screening applications. The synthesized PM-Chi-GO is characterized by its unique structural and biocompatible properties and holds significant promise for future drug screening endeavors. This study establishes a foundation for the utilization of this scaffold in drug screening applications.References:
[1] Muzzarelli, Riccardo AA., 1983, Chitin and its derivatives: new trends of applied research, Carbohydrate Polymers 3(1): 53-75. https://doi.org/10.1016/0144-8617(83)90012-7.
[2] Agnihotri, SA., Mallikarjuna, NN., Aminabhavi, TM., 2004, Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release, 100(1), 5–28,. https://doi.org/10.1016/j.jconrel.2004.08.010.
[3] Thomas, S., Pius, A., Gopi, S., 2020, Handbook of Chitin and Chitosan: Volume 1: Preparation and Properties. Elsevier, 1-33, https://doi.org/10.1016/B978-0-12-817970-3.00001-8.
[4] Duraisam, R., Ganapathy, D., Shanmugam, R., 2021, Applications of Chitosan in Dental Implantology - A Literature Review. International Journal of Dentistry and Oral Science (IJDOS), 8, 4140–6.
[5] Raslan, A., Saenz Del Burgo, L., Ciriza, J., Pedraz, JL., 2020, Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine, Int. J. Pharm, 580, 119226.https://doi.org/10.1016/j.ijpharm.2020.119226.
[6] Nasim, I., Rajesh Kumar, S., Vishnupriya, V., Jabin, Z., 2020, Cytotoxicity and anti-microbial analysis of silver and graphene oxide bio nanoparticles, Bioinformation 16, 831–6.
[7] Nasim, I., Rajeshkumar, S., Vishnupriya, V., 2021, Green synthesis of reduced graphene oxide nanoparticles, its characterization and antimicrobial properties against common oral pathogens. Int J Dentistry Oral Sci,8,1670–5.
[8] Gao, W., 2015, Graphene Oxide: Reduction Recipes, Spectroscopy, and Applications. Springer.
[9] Aiswaria, P., Naina Mohamed, S., Singaravelu, DL., Brindhadevi, K., Pugazhendhi, A., 2022, A review on graphene / graphene oxide supported electrodes for microbial fuel cell applications: Challenges and prospects. Chemosphere, 296, 133983.
[10] Georgakilas, V., ed 2014, Functionalization of Graphene, John Wiley & Sons.
[11] Subramanian, AK., Prabhakar, R., Vikram, NR., Dinesh, SS., Rajeshkumar, S., 2022, In vitro anti-inflammatory activity of silymarin/hydroxyapatite/chitosan nanocomposites and its cytotoxic effect using brine shrimp lethality assay, J. Popul. Ther. Clin. Pharmacol, 28, e71–7.
[12] Gama, M., Gatenholm, P., Klemm, D., 2016, Bacterial NanoCellulose: A Sophisticated Multifunctional Material. CRC Press.
[13] N’deh, KPU., Kim, GJ., Chung, KH., Shin, JS., Lee, KS., Choi, JW., et al, 2020, Surface-Modified Industrial Acrylonitrile Butadiene Styrene 3D Scaffold Fabrication by Gold Nanoparticle for Drug Screening, Nanomaterials, 10 (3), 529, http://dx.doi.org/10.3390/nano10030529
[14] Francis, AP., Gurudevan, S., Jayakrishnan, A., 2018, Synthetic polymannose as a drug carrier: synthesis, toxicity and anti-fungal activity of polymannose-amphotericin B conjugates, J. Biomater. Sci. Polym. Ed, 29, 1529–48.
[15] Miguel Oliveira, J., Pina, S., Reis, RL., Roman, JS., 2018, Osteochondral Tissue Engineering: Nanotechnology, Scaffolding-Related Developments and Translation, Springer, Vol 1058.
[16] Rieshy, V., Priya, J., Arivarasu, L., Kumar, SR., 2020, Enhanced antimicrobial activity of herbal formulation mediated copper nanoparticles against clinical pathogens, Plant Cell Biotechnology and Molecular Biology, 21(53-54), 52-56.
[17] Rajeshkumar, S., Lakshmi, T., Tharani, M., 2021, Green synthesis of copper nanoparticles synthesized using black tea and its antibacterial activity against oral pathogens, Int. J. Dent. Oral Sci, 8, 4156–9.
[18] Thakur, VK., Thakur, MK., Kessler, MR., 2017, Handbook of Composites from Renewable Materials, Biodegradable Materials. John Wiley & Sons, vol 5.
[19] Mi, XJ., Choi, HS., Perumalsamy, H., Shanmugam, R., Thangavelu, L., Balusamy, SR., et al., 2022, Biosynthesis and cytotoxic effect of silymarin-functionalized selenium nanoparticles induced autophagy mediated cellular apoptosis via downregulation of PI3K/Akt/mTOR pathway in gastric cancer. Phytomedicine,99, 154014.
[20] Nasim, I., Jabin, Z., Kumar, SR., Vishnupriya, V., 2022, Green synthesis of calcium hydroxide-coated silver nanoparticles using Andrographis paniculata and Ocimum sanctum Linn. leaf extracts: An antimicrobial and cytotoxic activity, J. Conserv. Dent, 25, 369–74.
[21] Rajeshkumar, S., Lakshmi, T., 2021, Green synthesis of gold nanoparticles using kalanchoe pinnata and its free radical scavenging activity, Int J Dentistry Oral Sci, 8,2981–4.
[22] Campana-Filho, SP., de Almeida Pinto, LA., 2017, Chitosan-Based Materials and its Applications. Bentham Science Publishers, vol 3.
[23] Danhier, F., Ansorena, E., Silva, JM., Coco, R., Le Breton, A., Préat, V., 2012, PLGA-based nanoparticles: an overview of biomedical applications, J. Control. Release, 161(2), 505–22, https://doi.org/10.1016/j.jconrel.2012.01.043.
[24] Roy, S., Rhim, JW., 2021, Fabrication of bioactive binary composite film based on gelatin/chitosan incorporated with cinnamon essential oil and rutin, Colloids Surf. B Biointerfaces, 204, 111830. https://doi.org/10.1016/j.colsurfb.2021.111830.
[25] DeSimone, RW., Currie, KS., Mitchell, SA., Darrow, JW., Pippin, DA., 2004, Privileged structures: applications in drug discovery, Comb. Chem. High Throughput Screen, 7(5), 473–94. https://doi.org/10.2174/1386207043328544.
[26] Mao, JS., Cui, YL., Wang, XH., Sun, Y., Yin, YJ., Zhao, HM., et al., 2004, A preliminary study on chitosan and gelatin polyelectrolyte complex cytocompatibility by cell cycle and apoptosis analysis, Biomaterials, 25, 3973–81, https://doi.org/10.1016/j.biomaterials.2003.10.080
[27] Karthik EVG, Priya V (2021) Gayathri. R, Dhanraj Ganapathy. Health Benefits Of Annona Muricata-A Review. Int J Dentistry Oral Sci 8:2965–2967
[28] Priya DV, (2020) Knowledge and awareness on HIV/AIDS among college students in A university hospital setting. Int J Dent Oral Sci 1182–1186
[29] Prakash S, Balaji JN, Veeraraghavan VP, Mohan SK (2022) Telehealth: Is It a Post-COVID Reality in Early Diagnosis of Oral Cancer? J Contemp Dent Pract 23:1181–1182

