Argyreia nervosa Mitigates Insulin Resistance in Liver via IR/IRS-1 Mediated Signaling in Streptozotocin-Induced Type-2 Diabetic Rats
Abstract:
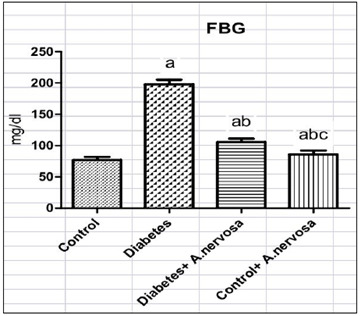
Argyreia nervosa, commonly known as Hawaiian baby woodrose or wooly morning glory, is a plant native to India and is also found in various parts of the world. It is known for its seeds, which have been used traditionally in Ayurvedic and traditional medicine systems for various diseases. The study was aimed at assessing the effect of Argyreia nervosa on insulin signaling molecules of the liver in streptozotocin (STZ)-induced experimental type-2 diabetic rats. Healthy adult male Wistar albino rats -150-180 days old weighing 180-200g was used for the study and divided as Group I - Normal rats; Group II – type-2 diabetic rats; Group III -Diabetic rats + A. nervosa 500 mg/kg b.wt; Group IV-Normal rats + A. nervosa 500 mg/kg b.wt. Fasting blood glucose, and fasting serum insulin were measured by calorimetric methods. Further mRNA expression analysis of insulin receptor (IR) was measured in control and treated animals by quantitative Ream Time PCR analysis. A. nervosa root extract significantly reduced fasting blood glucose and serum insulin concentrations in STZ-induced rats compared with control. In addition, mRNA expression of IR showed a one-fold increase in the expression which shows that A. nervosa is involved in the regulation of insulin signaling in the liver and thereby reduces insulin resistance and type-2 diabetes. Our study concludes that A. nervosa root extract has a significant role on insulin signaling molecules thereby it reduces hyperglycemia and hyperinsulinemia via insulin receptor-mediated pathways. Hence, A. nervosa may be considered as one of the therapeutic natural antidiabetic drugs.References:
[1] Vieira, R.F., Genetic Resources of Medicinal and Aromatic Plants from Brazil. Encyclopedia of Plant and Crop Science 2004; 502–505.
[2] Vishaka, S., Sridevi, G., & Selvaraj, J. (2022). An in vitro analysis on the antioxidant and anti-diabetic properties of Kaempferia galanga rhizome using different solvent systems. Journal of AdvancedPharmaceutical Technology and Research, 13 (6):505-509
[3] Bayat, E., Rahpeima, Z., Dastghaib, S., Gholizadeh, F., Erfani, M., Asadikaram, G., & Mokarram, P. (2020). Stevia rebaudiana extract attenuate metabolic disorders in diabetic rats via modulation of glucose transport and antioxidant signaling pathways and aquaporin-2 expression in two extrahepatic tissues. Journal of food biochemistry, 44(8), e13252. https://doi.org/10.1111/jfbc.13252
[4] Sneka, S, Sinduja, P., & Priyadharshini. (2022). A Comparative Study On Salivary Ph In Diabetic Patients With Periodontitis And Without Periodontitis. Journal of Pharmaceutical Negative Results, 2022, 13:1253–1258.
[5] Vikraman, K.S., Abilasha, & Kavitha, S. (2020). Knowledge about the effects of medicinal plants against COVID-19 among dental students-A questionnaire study. International Journal of Current Research and Review, 12: S97–S108.
[6] Warrier, V.P.K., Nambiar, & Ramankutty, C. (2011). Indian Medicinal Plants. A Compendium of 500 Species. https://search.worldcat.org/title/indian-medicinal-plants-a-compendium-of-500-species/oclc/29791294
[7] Salehi, B., Ata, A., V Anil Kumar, N., Sharopov, F., Ramírez-Alarcón, K., Ruiz-Ortega, A., Abdulmajid Ayatollahi, S., Tsouh Fokou, P. V., Kobarfard, F., Amiruddin Zakaria, Z., Iriti, M., Taheri, Y., Martorell, M., Sureda, A., Setzer, W. N., Durazzo, A., Lucarini, M., Santini, A., Capasso, R., Ostrander, E. A., & Sharifi-Rad, J. (2019). Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules, 9(10), 551. https://doi.org/10.3390/biom9100551.
[8] Priyadharshini, R., & Sinduja, P. (2022). Knowledge, Attitude, And Awareness Of Warning Signs And Risk Factor Of Liver Cirrhosis And Diabetes Mellitus Among Second Year Dental Students-A Survey. Journal of Pharmaceutical Negative Results,13: 1490–1499.
[9] Menon, G.R., Malaiappan, S., & Kumar, K. (2021). Association Between Right Upper Molar Involvement and Diabetes Mellitus In Subjects With Chronic Periodon-titis. International Journal of Dentistry and Oral Science, 8: 2879–2884.
[10] Paulke, A., Kremer, C., Wunder, C., Wurglics, M., Schubert-Zsilavecz, M., & Toennes, S.W. (2015). Studies on the alkaloid composition of the Hawaiian Baby Woodrose Argyreia nervosa, a common legal high. Forensic Sci. Int. 249, 281–293.
[11] Subramanyam, G.K., Gaddam, S.A., Kotakadi, V.S., Palithya, S., Penchalaneni, J., & Challagundla, V.N. (2021). Argyreia nervosa (Samudra pala) leaf extract mediated silver nanoparticles and evaluation of their antioxidant, antibacterial activity, in vitro anticancer and apoptotic studies in KB oral cancer cell lines. Artif. Cells Nanomed. Biotechnol. 1, 635–650.
[12] Singhal, A.K., Gupta, H., & Bhati, V.S. (2011). Wound healing activity of Argyreia nervosa leaves extract. Int. J. Appl. Basic Med. Res. 1:36–39.
[13] Milimita, P., Sujata, M., Janyanaranjan, P., & Nikunja, M.K. (2013). Traditional uses and phytopharmacological aspects of Argyreia nervosa. J. Adv. Pharm. Res. 4, 23–32.
[14] Meher, A., & Padhan, A.R. (2011). A Literature Review on Argyreia Nervosa (Burm. F.) Bojer. Int. J. Res. Ayurveda Pharm. 5, 1501–1504.
[15] Prasad, M., Jayaraman, S., Rajagopal, P., Veeraraghavan, V. P., Kumar, P. K., Piramanayagam, S., & Pari, L. (2022). Diosgenin inhibits ER stress-induced inflammation in aorta via iRhom2/TACE mediated signaling in experimental diabetic rats: An in vivo and in silico approach. Chemico-biological interactions, 358, 109885. https://doi.org/10.1016/j.cbi.2022.109885
[16] Roy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Veeraraghavan, V. P. (2022). Carica papaya Reduces Muscle Insulin Resistance via IR/GLUT4 Mediated Signaling Mechanisms in High Fat Diet and Streptozotocin-Induced Type-2 Diabetic Rats. Antioxidants (Basel, Switzerland), 11(10), 2081. https://doi.org/10.3390/antiox11102081.
[17] Khan, H. L. A., Sridevi, G., Selvaraj, J. and Preetha, S. (2021). In vitro Anti-inflammatory Properties in Various Extracts (Ethanol, Chloroform and Aqueous) of Kaempferia galanga Linn Rhizome”, Journal of Pharmaceutical Research International, 33(47B), 476–481. doi: 10.9734/jpri/2021/v33i47B33146.
[18] Prathap, L., Jayaraman, S., Roy, A., Santhakumar, P., & Jeevitha, M. (2021). Molecular docking analysis of stachydrine and sakuranetin with IL-6 and TNF-α in the context of inflammation. Bioinformation, 17(2), 363–368. https://doi.org/10.6026/97320630017363.
[19] Roy, J. R., Janaki, C. S., Jayaraman, S., Veeraraghavan, V. P., Periyasamy, V., Balaji, T., Vijayamalathi, M., Bhuvaneswari, P., & Swetha, P. (2023). Hypoglycemic Potential of Carica papaya in Liver Is Mediated through IRS-2/PI3K/SREBP-1c/GLUT2 Signaling in High-Fat-Diet-Induced Type-2 Diabetic Male Rats. Toxics, 11(3), 240. https://doi.org/10.3390/toxics11030240.
[20] Mithil Vora., Vishnu Priya, V., Selvaraj,J., Gayathri, R., & Kavitha, S. (2021). Effect of Lupeol on proinflammatory Markers in Adipose Tissue of High-Fat Diet and Sucrose Induced Type-2 Diabetic Rats. Journal of Research in Medical and Dental Science, 9(10):116-121.
[21] Salehi, B., Ata, A., V Anil Kumar, N., Sharopov, F., Ramírez-Alarcón, K., Ruiz-Ortega, A., Abdulmajid Ayatollahi, S., Tsouh Fokou, P. V., Kobarfard, F., Amiruddin Zakaria, Z., Iriti, M., Taheri, Y., Martorell, M., Sureda, A., Setzer, W. N., Durazzo, A., Lucarini, M., Santini, A., Capasso, R., Ostrander, E. A., Sharifi-Rad, J. (2019). Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules, 9(10), 551. https://doi.org/10.3390/biom9100551.
[22] Jayaraman, S., Krishnamoorthy, K., Prasad, M., Veeraraghavan, V.P., Krishnamoorthy, R., Alshuniaber, M.A., Gatasheh, M.K., Elrobh, & M., Gunassekaran. (2023). Glyphosate potentiates insulin resistance in skeletal muscle through the modulation of IRS-1/PI3K/Akt mediated mechanisms: An in vivo and in silico analysis. Int J Biol Macromol, 242(Pt 2):124917. doi: 10.1016/j.ijbiomac.2023.124917.
[23] Chandran, D., Jayaraman, S., Sankaran, K., Veeraraghavan, V. P., & R, G. (2023). Antioxidant Vitamins Attenuate Glyphosate-Induced Development of Type-2 Diabetes Through the Activation of Glycogen Synthase Kinase-3 β and Forkhead Box Protein O-1 in the Liver of Adult Male Rats. Cureus, 15(12), e51088. https://doi.org/10.7759/cureus.51088.
[24] Yasothkumar. D., Jayaraman, S., Ramalingam, K., & Ramani, P. (2023). In vitro Anti-Inflammatory and Antioxidant Activity of Seed Ethanolic Extract of Pongamia pinnata. Biomed Pharmacol J.16(4).
[25] Prasad, M., Jayaraman, S., Natarajan, S.R., Veeraraghavan, V.P., Krishnamoorthy, R., Gatasheh, M.K., Palanisamy, C.P., & Elrobh, M. (2023). Piperine modulates IR/Akt/GLUT4 pathways to mitigate insulin resistance: Evidence from animal and computational studies. Int J Biol Macromol, 253(Pt 5):127242. doi: 10.1016/j.ijbiomac.2023.127242.
[26] Jayaraman, S., Devarajan, N., Rajagopal, P., Babu, S., Ganesan, S.K., Veeraraghavan, V.P., Palanisamy, C.P., Cui, B., Periyasamy, V., & Chandrasekar K. (2021). β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules. 26(7), 2101. doi: 10.3390/molecules26072101.
[27] Kiruthigha, T., Gayathri, R., Vishnu Priya, V., Selvaraj Jayaraman, & Kavitha, S. (2023). Piperine Modulates High Fat Diet - Induced Renal Damage by Regulating Kim-1 and Igf-1 Beta Signaling Molecules in Male Wistar Rats”. Journal of Advanced Zoology, 44 (S5):246-54.
[28] Thana Lakshme, P.S., Gayathri, R., & Vishnu Priya V. (2021). Preliminary Phytochemical Screening and Estimation of Total Phenolic Content of Aqueous Cladode Extract of Opuntia dilleniid. Journal of Research in Medical and Dental Science, 9(2): 254-257.
[29] Kalaiselvi, K., Selvaraj, J., Rajapandiyan K., Salim, M.,, Mohammad, A., Alshuniaber, Biba V., & Vishnu Priya, V. (2023). Green synthesis and evaluation of anti-microbial, antioxidant, anti-inflammatory, and anti-diabetic activities of silver nanoparticles from Argyreia nervosa leaf extract: An invitro study. Journal of King Saud University – Science, 35(10):102955.
[30] Ojastha, B.L., Selvaraj, J., Kavitha, S., Veeraraghavan Vishnu Priya., & Gayathri. ( Effect Of Argyreia Nervosa On The Expression Of Growth Factor Signaling In The Skeletal Muscle Of Streptozotocin-Induced Experimental Diabetic Rats. ournal of Namibian Studies: History Politics Culture, 33: 5942-5950. https://doi.org/10.59670/jns.v33i.4474.
[31] Karthik EVG, Priya V (2021) Gayathri. R, Dhanraj Ganapathy. Health Benefits Of Annona Muricata-A Review. Int J Dentistry Oral Sci 8:2965–2967
[32] Priya DV, (2020) Knowledge and awareness on HIV/AIDS among college students in A university hospital setting. Int J Dent Oral Sci 1182–1186
[33] Prakash S, Balaji JN, Veeraraghavan VP, Mohan SK (2022) Telehealth: Is It a Post-COVID Reality in Early Diagnosis of Oral Cancer? J Contemp Dent Pract 23:1181–1182
[34] Ealla KKR, Veeraraghavan VP, Ravula NR, Durga CS, Ramani P, Sahu V, Poola PK, Patil S, Panta P (2022) Silk Hydrogel for Tissue Engineering: A Review. J Contemp Dent Pract 23:467–477
[35] Patil S, Sujatha G, Varadarajan S, Priya VV (2022) A bibliometric analysis of the published literature related to toothbrush as a source of DNA. World J Dent 13:S87–S95

