Molecular Approach to Identify Anti-inflammatory Potential of Stevioside in HFD-induced Type 2 Diabetic Rats: Evidence From in Vivo Study
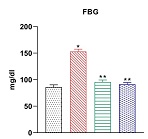
Abstract:
References:
[1] DeFronzo, R. A., Ferrannini, E., Groop, L., Henry, R. R., Herman,
W. H., Holst, J. J., Hu, F. B., Kahn, C. R., Raz, I., Shulman, G. I., Simonson,
D. C., Testa, M. A., & Weiss, R. (2015). Type 2 diabetes mellitus. Nature
Reviews Disease Primers, 1(1), 15019. https://doi.org/10.1038/nrdp.2015.19.
[2]
Guariguata, L., Whiting, D.,
Weil, C., & Unwin, N. (2011). The International Diabetes Federation diabetes
atlas methodology for estimating global and national prevalence of diabetes in adults.
Diabetes Research and Clinical Practice, 94(3), 322–332. https://doi.org/10.1016/j.diabres.2011.10.040.
[3] Hu, F. B. (2011). Globalization of Diabetes. Diabetes Care,
34(6), 1249–1257. https://doi.org/10.2337/dc11-0442.
[4]
Prasad, M., Rajagopal, P.,
Devarajan, N., Veeraraghavan. V.P., Palanisamy, C.P., Cui, B., Patil, S., &
Jayaraman, S. (2022). A comprehensive review on high -fat diet-induced diabetes
mellitus: an epigenetic view. J Nutr Biochem. 107:109037. doi: 10.1016/j.jnutbio.2022.109037.
[5] Kiruthigha, T., Gayathri, R., Vishnu Priya, V., Selvaraj Jayaraman,
& Kavitha, S. (2023). Piperine
Modulates High Fat Diet - Induced Renal Damage by Regulating Kim-1 and Igf-1 Beta
Signaling Molecules in Male Wistar Rats”. Journal of Advanced Zoology, 44
(S5):246-54.
[6]
Dandona, P. (2004). Inflammation:
the link between insulin resistance, obesity, and diabetes. Trends in Immunology,
25(1), 4–7. https://doi.org/10.1016/j.it.2003.10.013.
[7] Tsalamandris, S., Antonopoulos, A. S., Oikonomou, E., Papamikroulis,
G.-A., Vogiatzi, G., Papaioannou, S., Deftereos, S., & Tousoulis, D. (2019).
The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives.
European Cardiology Review, 14(1), 50–59. https://doi.org/10.15420/ecr.2018.33.1.
[8]
Cruz, N. G., Sousa, L. P.,
Sousa, M. O., Pietrani, N. T., Fernandes, A. P., & Gomes, K. B. (2013). The
linkage between inflammation and Type 2 diabetes mellitus. Diabetes Research
and Clinical Practice, 99(2), 85–92. https://doi.org/10.1016/j.diabres.2012.09.003.
[9] Lontchi-Yimagou, E., Sobngwi, E., Matsha, T. E., & Kengne,
A. P. (2013). Diabetes Mellitus and Inflammation. Current Diabetes Reports,
13(3), 435–444. https://doi.org/10.1007/s11892-013-0375-y.
[10]
Padmapriya, A., Preetha, S.,
Selvaraj, J., & Sridevi, G. (2022). Effect of Carica papaya seed extract on
IL-6 and TNF-α in human lung cancer cell lines-an In vitro study. Research Journal
of Pharmacy and Technology, 15 (12): 5478-5482.
[11]
Nath, S., Ghosh, S. K., &
Choudhury, Y. (2017). A murine model of type 2 diabetes mellitus developed using
a combination of high fat diet and multiple low doses of streptozotocin treatment
mimics the metabolic characteristics of type 2 diabetes mellitus in humans. Journal
of Pharmacological and Toxicological Methods, 84, 20–30. https://doi.org/10.1016/j.vascn.2016.10.007.
[12]
Herieka, M., & Erridge,
C. (2014). High‐fat meal induced postprandial inflammation. Molecular Nutrition
& Food Research, 58(1), 136–146. https://doi.org/10.1002/mnfr.201300104.
[13] Mounithaa, N., Gayathri, R., Selvaraj
Jayaraman, Vishnu Priya, V., & Kavitha, S. (2023). Effect of Piperine on an
Nrf2/Keap 1 Signalling Mechanism in Adipose Tissue of High Fat Diet and Sucrose-Induced
Experimental Diabetic Rats. Journal of Advanced
Zoology, 44 (S5):232-39.
[14] Modak, M., Dixit, P., Londhe, J., Ghaskadbi, S., & Devasagayam,
T. P. A. (2007). Indian Herbs and Herbal Drugs Used for the Treatment of Diabetes.
Journal of Clinical Biochemistry and Nutrition, 40(3), 163–173. https://doi.org/10.3164/jcbn.40.163.
[15]
Pang, G.-M., Li, F.-X., Yan,
Y., Zhang, Y., Kong, L.-L., Zhu, P., Wang, K.-F., Zhang, F., Liu, B., & Lu,
C. (2019). Herbal medicine in the treatment of patients with type 2 diabetes mellitus.
Chinese Medical Journal, 132(1), 78–85. https://doi.org/10.1097/CM9.0000000000000006.
[16]
Thana Lakshme, P.S., Gayathri,
R., & Vishnu Priya V. (2021). Preliminary Phytochemical Screening and Estimation
of Total Phenolic Content of Aqueous Cladode Extract of Opuntia dilleniid. Journal of Research in Medical and Dental Science,
9(2): 254-257.
[17]
Mithil Vora, Vishnu Priya,
V., Selvaraj,J., Gayathri, R., & Kavitha, S. (2021). Effect of Lupeol on proinflammatory
Markers in Adipose Tissue of High-Fat Diet and Sucrose Induced Type-2 Diabetic Rats.
Journal of Research in Medical and Dental
Science, 9(10):116-121.
[18]
Vishaka, S., Sridevi, G.,
& Selvaraj, J. (2022). An in vitro
analysis on the antioxidant and anti-diabetic properties of Kaempferia galanga rhizome using different
solvent systems. Journal of Advanced Pharmaceutical
Technology and Research, 13 (6): 505-509.
[19] Chen, T.-H., Chen, S.-C., Chan, P., Chu, Y.-L., Yang, H.-Y., &
Cheng, J.-T. (2005). Mechanism of the Hypoglycemic Effect of Stevioside, a Glycoside
of Stevia rebaudiana. Planta Medica, 71(2), 108–113. https://doi.org/10.1055/s-2005-837775.
[20] Orellana-Paucar, A. M. (2023). Steviol Glycosides from Stevia
rebaudiana: An Updated Overview of Their Sweetening Activity, Pharmacological Properties,
and Safety Aspects. Molecules, 28(3), 1258. https://doi.org/10.3390/molecules28031258.
[21]
Dev Arora, Gayathri, R., Selvaraj,
J., Vishnu Priya, V., & Kavitha, S. (2021). Vitamin C and E Down Regulates the
Expression of C-JNK, IKKB, NF-kB in Adipose Tissue of PCB-Exposed Rats. Journal of Research in Medical and Dental Science,
9(11):39-44.
[22]
Khan, H.L.A., Sridevi, G.,
Selvaraj, & J. Preetha, S. (2021). In
vitro Anti-inflammatory Properties in Various Extracts (Ethanol, Chloroform
and Aqueous) of Kaempferia galanga Linn
Rhizome. Journal of Pharmaceutical Research International, 33 (47B): 476–481. DOI:https://doi.org/10.9734/jpri/2021/v33i47B33146.
[23]
Ponnulakshmi,
R., Shyamaladevi, B., Vijayalakshmi, P., & Selvaraj, J. (2019). In silico and
in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose
tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicology
mechanisms and methods, 29(4), 276–290. https://doi.org/10.1080/15376516.2018.1545815.
[24] Jayaraman, S., Devarajan, N., Rajagopal, P., Babu, S., Ganesan,
S.K., Veeraraghavan, V.P., Palanisamy, C.P., Cui, B., Periyasamy, V., & Chandrasekar
K. (2021). β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance
by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2
Diabetic Rats. Molecules. 26(7), 2101.
doi: 10.3390/molecules26072101.
[25] Fan, C., Song, Q., Wang, P., Li, Y., Yang, M., & Yu, S.Y.
(2019). Neuroprotective Effects of Curcumin on IL-1β-Induced Neuronal Apoptosis
and Depression-Like Behaviors Caused by Chronic Stress in Rats. Frontiers in Cellular Neuroscience, 7, 12:516.
doi: 10.3389/fncel.2018.00516.
[26]
Zhang, M., Lv, X.-Y., Li,
J., Xu, Z.-G., & Chen, L. (2008). The Characterization of High-Fat Diet and
Multiple Low-Dose Streptozotocin Induced Type 2 Diabetes Rat Model. Experimental
Diabetes Research, 2008, 1–9. https://doi.org/10.1155/2008/704045.
[27]
Nabi, S. A., Kasetti, R. B.,
Sirasanagandla, S., Tilak, T. K., Kumar, M. V. J., & Rao, C. A. (2013). Antidiabetic
and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced
diabetic rats. BMC Complementary and Alternative Medicine, 13(1),
37. https://doi.org/10.1186/1472-6882-13-37.
[28] Jayaraman, S., Krishnamoorthy, K.,
Prasad, M., Veeraraghavan, V.P., Krishnamoorthy, R., Alshuniaber, M.A., Gatasheh,
M.K., Elrobh, & M., Gunassekaran. (2023).
Glyphosate potentiates insulin resistance in skeletal muscle through the modulation
of IRS-1/PI3K/Akt mediated mechanisms: An in vivo and in silico analysis. Int J Biol Macromol, 242(Pt 2):124917. doi:
10.1016/j.ijbiomac.2023.124917.
[29] Prasad, M., Jayaraman, S., Natarajan,
S.R., Veeraraghavan, V.P., Krishnamoorthy, R., Gatasheh, M.K., Palanisamy, C.P.,
& Elrobh, M. (2023). Piperine modulates IR/Akt/GLUT4 pathways to mitigate insulin
resistance: Evidence from animal and computational studies. Int J Biol Macromol,
253(Pt 5):127242. doi: 10.1016/j.ijbiomac.2023.127242.
[30]
Shen, P., Liu, M., Ng, T.,
Chan, Y., & Yong, E. L. (2006). Differential Effects of Isoflavones, from Astragalus
Membranaceus and Pueraria Thomsonii, on the Activation of PPARα, PPARγ, and Adipocyte
Differentiation In Vitro. The Journal of Nutrition, 136(4), 899–905.
https://doi.org/10.1093/jn/136.4.899.
[31]
Akifa Begum, Palati Sinduja,
Priyadharshini, R., & Selvaraj Jayaraman. (2021). Estimation of Clinocopathological
Correlation and Comparison of Salivary TNF-α among Normal and Post Radiotherapy
Patients of Oral cancer-A Cross-Sectional Study. Journal of Research in Medical and Dental Science, 9(10): 92-97.
[32] Fathima Hinaz, Z., Gayathri, R., Selvaraj,
J., Vishnu Priya, V., Kavitha, S., & Gayathri, R. (2021). Comparative Evaluation
of Anti-Cholesterol Potential of Apple Cider Vinegar and Its Herbal Formulation
with Allium Sativum and Honey-An In-Vitro Assay. Journal of Research in Medical
and Dental Science 9 (10),142-147.
[33] Picard, F., & Auwerx, J. (2002). PPARγ and glucose homeostasis.
Annual Review of Nutrition, 22(1), 167–197. https://doi.org/10.1146/annurev.nutr.22.010402.102808.
[34] Staels, B., & Fruchart, J.-C. (2005). Therapeutic Roles of
Peroxisome Proliferator–Activated Receptor Agonists. Diabetes, 54(8),
2460–2470. https://doi.org/10.2337/diabetes.54.8.2460.
[35] Daynes, R. A., & Jones, D. C. (2002). Emerging roles of PPARS
in inflammation and immunity. Nature Reviews Immunology, 2(10), 748–759.
https://doi.org/10.1038/nri912.
[36]
Sharma, B., Salunke, R., Srivastava,
S., Majumder, C., & Roy, P. (2009). Effects of guggulsterone isolated from Commiphora
mukul in high fat diet induced diabetic rats. Food and Chemical Toxicology,
47(10), 2631–2639. https://doi.org/10.1016/j.fct.2009.07.021.

