Effectiveness of Mobile Phone Reminders in Improving Adherence and Treatment Outcomes of Patients on Art in Adamawa State, Nigeria: A Ramdomized Controlled Trail
Abstract:
Adherence to antiretroviral therapy
(ART) among people living with human immunodeficiency virus (PLHIV) is very imperative
in achieving successful treatment outcome and decreased risk of HIV transmission
to uninfected people. This is a randomized controlled
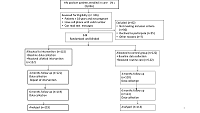
trial study conducted in Adamawa State, Nigeria. 244 patients were randomized to
intervention or control group. Data
obtained from the study was analyzed using SPSS Version 21. Frequencies distributions,
descriptive statistics were presented, Inferential statistics such as Pearson Chi
square, McNemar’s test, Paired T test, correlation and repeated measures ANOVA were
used to measure the strength of associations and relationships between the various
variables and probability of statistically significant level set < 0.05 at 95%
Confidence interval. The response rates in the intervention and control groups were
99% and 96.7% at 3 months; 97.5% and 92.6% at 6 months, respectively. Individual
socio-demographic characteristics were not found to be associated with adherence
levels in this study. At six months follow up the proportion of the respondents
who had good adherence (>95%) was higher (89.1%) and statistically significant
(p= 0.001) in the intervention group compared to control group (63.1%) and (p= 0.617).
A significantly higher frequency in missed clinic appointments (7.98 vs 1.68) (p=0.024)
was noticed in the control group, and a statistically significant increase in the
proportion of participants who reported an increase in weight (p=0.001), CD4 cells
counts (p=0.001) and decrease in the presence of tuberculosis and other opportunistic
infections were observed among patients in the intervention group.
References:
[1]
Joint
United Nation Programme on HIV/AIDS (UNAIDS, 2018). Global HIV & AIDS statistics.
2018 fact sheet. http://www.unaids.org/en/resources/fact-sheet).
[3]
National
HIV Sero-prevalence Sentinel Survey (NHSS 2014).
[4]
Bekele
Belayihun1 and Rahma Negus (2015). Antiretroviral Treatment Adherence Rate and Associated
Factors among People Living with HIV in Dubti Hospital, Afar Regional State, East
Ethiopia. Hindawi Publishing Corporation International Scholarly Research Notices
Volume 2015, Article ID 187360, 5 pages. http://dx.doi.org/10.1155/2015/187360.
[5]
World
Health Organization. (WHO, 2005) Interim WHO clinical staging of HIV/AIDS and HIV/AIDS
case definitions for surveillance: African region. Switzerland: https://www.who.int/hiv/pub/guidelines/clinicalstaging.pdf.
[6]
Paterson
DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. (2002) Adherence to protease inhibitor therapy and outcomes in
patients with HIV infection. Annals of internal medicine. 2000;133(1):21–30. pmid:10877736.
[7]
Suleiman
IA, Momo A. Adherence to antiretroviral therapy and its determinants among persons
living with HIV/AIDS in Bayelsa state, Nigeria. Pharmacy Practice 2016 Jan-Mar;14(1):631.
doi: 10.18549/PharmPract.2016.01.631.
[8]
Kelley
LL' Engle, Kimberly Green MA, Stacey M Succop et al., (2015). Scaled-Up Mobile Phone Intervention for HIV Care and
Treatment: Protocol for a Facility Randomized Controlled Trial. JMIR Res Protoc
2015;4(1): e11) doi:10.2196/resprot.3659 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4319075/.
[9] National Guideline
for HIV Prevention Treatment and Care (2016)- Nigeria. http://apps.who.int/medicinedocs/documents/s23252en/s23252en.pdf.
[10]
President
Emergency Plan for AIDS Relief (PEPFAR) (2016). Country/Regional Operational Plan
(COP/ROP) 2016 guidance. Office of the U.S. Global AIDS Coordinator, Washington,
D.C.: OGAC;2015.
[11]
World
Health Organization (WHO, 2015). Guideline on when to start antiretroviral therapy
and on pre-exposure prophylaxis for HIV. Geneva: World Health Organization 2015
(http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en.
[12]
Lemeshow
S, David W. Ho, J Klar, S K. Lwanga and World Health Organization (1990). Adequacy
of sample size in health studies. WHO IRIS: 239. http://www.who.int/irs/handle/10665/41607.accessed.
[13]
Olowookere,
S.A., Fatiregun, A.A, Ladipo, M.M.A, Abioye-Kuteyi, E.A. & Adewole, I.F. (2016).
Effects of adherence to antiretroviral therapy on body mass index, immunological
and virological status of Nigerians living with HIV/AIDS. Alexandria Journal of
Medicine (2016) 52, 51–54.
[14]
Anoje,
C., Agu, K.A., Oladele, E.A., Badru, T., Adedokun, O., Oqua, D., Khamofu, H., Adebayo,
O., Torpey, K., Chabikuli, O.N. (2017). Adherence to On-Time ART Drug Pick-Up and
Its Association with CD4 Changes and Clinical Outcomes Amongst HIV Infected Adults
on First-Line Antiretroviral Therapy in Nigerian Hospitals AIDS Behav. 2017 Feb;21(2):386-392.
doi: 10.1007/s10461-016-1473-z.
[15]
Applebaum
AJ, Richardson MA, Brady SM, Brief DJ, keane TM (2009). Gender and Other psychosocial
factors as predictors of adherence to HAART in adults with comorbid HIV/aids, psychiatric
and substance related disorder. Aids Behav. ;13(1):60–5.
[16]
Carrieri
MP, Chesney MA, Spire B, et al. (1999).
Failure to maintain adherence to HAART in a cohort of French HIV-positive injecting
drug users. Int J Behav Med. 2003; 10:1–14.8. Holzemer WL, Corless IB, Nokes KM,
et al (2000). Predictors of self-reported adherence in persons living with HIV disease.
AIDS Patient Care STDS.13:185–97.9.
[17]
Bouhnik
AD, Chesney M, Carrieri P, et al. (2002)
Non adherence among HIV-infected injecting drug users: the impact of social instability.
J Acquire Immune Defic Syndr. ;31(suppl 3): S149 –S153.10.
[18]
Golin
CE, Liu H, Hays RD, et al. (2002). A prospective
study of predictors of adherence to combination antiretroviral medication. J Gen
InternMed. 2002; 17:756 – 65.11.
[19]
Gordillo
V, del Amo J, Soriano V, Gonzalez-Lahoz J. (1999) Socio demo-graphic and psychological
variables influencing adherence to antiretroviral therapy. AIDS.13:1763–9.12.
[20]
Chesney,
M.A. (2000). Factors affecting adherence to antiretroviral therapy. Clinical Infectious
Disease 2000; 30 S171–S76.
[21]
Eldred
LJ, Wu AW, Chaisson RE, Moore RD (1998) Adherence to antiretroviral and pneumocystis
prophylaxis in HIV disease. J Acquired Immune Defic Syndr Hum Retrovirol.;
18:117–25.5.
[22] Moatti J.P, Carrieri
M.P, Spire B, Gastaut J.A, Cassuto J.P, Moreau J. (2000) Adherence to HAART in French
HIV-infected injecting drug users: the contribution of buprenorphine drug maintenance
treatment. The Manif 2000 study group. AIDS. 2000; 14:151–5.6.
[23]
Wagner,
G.J. (2002). Predictors of antiretroviral adherence as measured by self-report,
electronic monitoring, and medication diaries. AIDS Patient Care STDS. 16:599 –
608.
[24] Nduaguba, S.O., Soremekun, R.O., Olugbake, O.A., & Barner, J.C (2017).
The relationship between patient-related factors and medication adherence among
Nigerian patients taking highly active anti-retroviral therapy. Africa Health Science.
17(3): 738–745.
[25]
Bonolo,
P.F., Ceccato, M.B., Rocha, G. M., Acúrcio, F.A., Campos, L. N., & Guimarães,
M. C. (2013). Gender differences in non-adherence among Brazilian patients initiating
antiretroviral therapy. Clinics (Sao Paulo). 68, 612–620.
[26]
Simoni,
J.M., Pearson, C.R., Pantalone, D.W., et al.
(2006) Efficacy of interventions in improving highly active antiretroviral therapy
adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled
trials. Journal Acquired Immune Deficiency Syndrome 43(Suppl 1): S23–S35.
[27]
Wagner,
G.J, Kanouse, D.E., Golinelli, D., et al
(2006). Cognitive-behavioral intervention to enhance adherence to antiretroviral
therapy: a randomized controlled trial (CCTG 578) AIDS 20:1295–1302.
[28]
Carrico,
A.W, Antoni, M.H., Duran, R.E., et al.
(2006). Reductions in depressed mood and denial coping during cognitive behavioral
stress management with HIV-positive gay men treated with HAART. Annual Behavior
Medicine 31:155–164.
[29]
Pop-Eleches
C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque D, et al. (2011). Mobile phone technologies
improve adherence to antiretroviral treatment in a resource-
limited setting: a randomized controlled trial of text message reminders. AIDS.
2011;25(6):825–34. pmid:21252632.
[30]
Lester
RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. (2010). Effects of a mobile phone short message service on antiretroviral
treatment adherence in Kenya (WelTel Kenya1): a randomized trial. Lancet. 2010;376(9755):1838–45.
pmid:21071074 [doi:10.1016/S0140-6736(10)61997-6].
[31]
Woods
SP, Moran LM, Carey CL, Dawson MS, Iudicello JE, et al. (2008) Prospective memory in HIV infection: is ‘‘remembering
to remember’’ a unique predictor of self-reported medication management? Arch Clin
Neuropsychology 23:257–270.
[32]
Chung
MH, Richardson BA, Tapia K, Benki-Nugent S, Kiarie JN, Simoni JM, et al. (2011). A randomized controlled trial
comparing the effects of counseling and alarm device on HAART adherence and virologic
outcomes. PLoS Medicine. 2011;8(3): e1000422. pmid:21390262.
[33]
Abdulrahman
SA, Rampal L, Ibrahim F, Radhakrishnan AP, Kadir Shahar H, Othman N (2017) Mobile
phone reminders and peer counseling improve adherence and treatment outcomes of
patients on ART in Malaysia: A randomized clinical trial. PLoS ONE 12(5): e0177698.
https://doi.org/10.1371/journal.pone.0177698.
[34]
Kunutsor S, Walley J, Katabira E, Muchuro S, Balidawa
H, Namagala E, et al. Using mobile phones
to improve clinic attendance amongst an antiretroviral treatment cohort in rural
Uganda: A cross-sectional and prospective study. AIDS Behav. 2010; 14(6): 1347±1353.
https://doi.org/10.1007/s10461-010-9780-2 PMID:
20700644.
[35]
Sarna
A, Luchters S, Geibel S, Chersich MF, Munyao P, Kaai S, et al. Short-and long-term efficacy of modified directly observed antiretroviral
treatment in Mombasa, Kenya: a randomized trial. J Acquir Immune Defic Syndr. 2008;
48(5), 611±619. https://doi.org/10.1097/QAI.0b013e3181806bf1 PMID:18645509.
[36]
Rich
ML, Miller AC, Niyigena P, Franke MF, Niyonzima JB, Socci A, et al. (2012). Excellent Clinical Outcomes
and High Retention in Care among Adults in a Community-Based HIV Treatment Program
in Rural Rwanda J Acquire Immune Defic Syndr. 2012; 59(3): e35±e42. https://doi.org/10.1097/QAI.0b013e31824476c4
PMID: 22156912.
[37]
Mannheimer S, Friedland G, Matts J, Child C, Chesney
M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes
for human immunodeficiency virus infected persons in clinical trials. Clin Infect
Dis. 2002; 34:1115–21.
[38]
El-Khatib
Z, Ekstrom AM, Coovadia A, Abrams EJ, Petzold M, et al. (2011) Adherence and virologic suppression during the first 24
weeks on antiretroviral therapy among women in Johannesburg, South Africa - a prospective
cohort study. BMC Public Health 11: 88.
[39]
Sampaio-Sa
M, Page-Shafer K, Bangsberg DR, et al.
100% adherence study: educational workshops vs video sessions to improve adherence
among ART-naive patients in Salvador, Brazil. AIDS Behaviour. 2008;12(4 Suppl):
S54–S62.
[40]
Garcia
R, Ponde M, Lima M, Souza AR, Stolze SM, Badaro R. Lack of effect of motivation
on the adherence of HIV-positive/AIDS patients to antiretroviral treatment. Braz
J Infect Dis. 2005;9(6):494–499.

