Table of Contents - Issue

July 2017
Volume 2 | Issue 1
In this issue, we are going to see the molecular mechanisms involved in tissues to harmful stimuli, Characteristic analysis of Caralluma attenuata extract, Sacral hiatus, study on local anesthetic eutectic mixture in New Zealand white rabbits, Neuroprotective Epigenetic and DNA Damage repairing molecular mechanisms and also about Antifungal, contraceptive, anti-cancer, mosquito repellent properties of Azadirachtaindica.
ISSUE DOI: 10.21522./TIJBMS.2016.02.01
[Download Full Issue]Recent articles
-
 Molecular Mechanisms Involved in Inflammatory Cascade: A ReviewAuthor: Jagan NadipellyDOI: 10.21522./TIJBMS.2016.02.01.Art001
Molecular Mechanisms Involved in Inflammatory Cascade: A ReviewAuthor: Jagan NadipellyDOI: 10.21522./TIJBMS.2016.02.01.Art001Molecular Mechanisms Involved in Inflammatory Cascade: A Review
Abstract:
Inflammation participates importantly in host defenses against infectious agents and injury, but it also contributes to the pathophysiology of many chronic diseases. Many molecular mechanisms are involved in the process of inflammation such as Prostaglandins, Platelet activating factors, Leukotrienes, Tumor necrosis factor alpha (TNFα), Interleukin one beta (IL1β), nuclear factor kappa beta (NFΚβ) and oxidative stress. These inflammatory molecules work together in concert to produce inflammation. The therapeutic targets for resolving inflammation are numerous because the process of inflammation is multifaceted. Inflammatory mediators, free radical activity and oxidative stress have been found to be attractive anti-inflammatory targets. The role of these components must be understood in order to effectively investigate inflammatory mediators as drug targets. This review mainly aims to summarise our current understanding of the molecular basis of inflammation and therapeutic implications to prevent this phenomenal activity.
Keywords: Inflammation, Bradykinin, Adenosine triphosphate, Tumor necrosis factor, nitric oxide synthase, Prostaglandins, nuclear factor kappa beta.
Molecular Mechanisms Involved in Inflammatory Cascade: A Review
References:
[1]. Akkol, Das S., Sarker, SD. and Nahar L. (2012). The treatment of inflammation, pain, and fever using medicinal plants. Advances in Pharmacological Sciences. 2012: 476985.
[2]. Arif Zaidi, SM., Khatoon, K and Aslam, KM. (2012). Role of herbal medicine in Ussuruttams (Dysmenorrhoea). Journal of Academic and Industrial Research. 1: 113-117.
[3]. Arima, M. and Fukuda, T. (2011). Prostaglandin D2 and TH2 inflammation in the pathogenesis of bronchial asthma. Korean Journal of Internal Medicine. 26: 8-18.
[4]. Austin PJ, Moalem-Taylor G. Pathophysiology of neuropathic pain: inflammatory mediators. In Toth C, Moulin DE, (editors). Neuropathic Pain. Cambridge University Press: New York; 2013 ;( 7):77-89.
[5]. Bai, H. and Zhu, BT. (2008). Strong activation of cyclooxygenase I and II catalytic activity by dietary bioflavonoids. Journal of Lipid Research. 49: 2557–2570.
[6]. Biller-Takahashi, JD, Takahashi, LS, Saita, MV, Gimbo RY and Urbinati, EC. (2013). Leukocytes respiratory burst activity as an indicator of innate immunity of pacu Piaractus mesopotamicus. Brazillian Journal of Biology. 73: 425-429.
[7]. Boukhatem, MN. Kameli, A., Ferhat, MA, Saidi, F. and Mekarnia M. (2013). Rose geranium essential oil as a source of new and safe anti-inflammatory drugs. Libyan Journal of Medicine. 8: 10.
[8]. Bulbul, IJ, Near, L. and Haque M. (2011). Antibacterial, cytotoxic and antioxidant activity of chloroform, n-hexane and ethyl acetate extract of plant Coccinia cordifolia. Agriculture and Biology Journal of North America. 2: 713-719.
[9]. Burk, DR., Cichacz, ZA., and Daskalova SM. (2010). Aqueous extract of Achillea millefolium L. (Asteraceae) inflorescences suppresses lipopolysaccharide-induced inflammatory responses in RAW 264.7 murine macrophages. Journal of Medicinal Plants Research. 4: 225-234.
[10]. Charles, D. J. (2013). Antioxidant assays. In: Antioxidant Properties of Spices, Herbs and Other Sources. Springer Science + Business Media, New York, 9-38.
[11]. Cheenpracha, S., Park, E., Rostama, B., Pezzuto, JM., and Chang, LC. (2010). Inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 cells by the norsesterterpene peroxide, epimuqubilin A. Marine Drugs. 8: 429-437.
[12]. Chen, ZJ. (2005). Ubiquitin signaling in the NF-Κβ pathway. Nature Cell Biology. 7: 758-765.
[13]. Das Sarma, A., Mallick, AR. and Ghosh AK. (2010). Free radicals and their role in different clinical conditions: An overview. International Journal of Pharma Sciences and Research. 1:
[14]. 185-192.
[15]. Dawes JM, Anderson DA, Bennett DL, Bevan S, McMahon SB. Inflammatory mediators and modulators of pain. Wall and Melzack’s Textbook of Pain. 2013; 6:48-67
[16]. Franceschelli, S., Pesce, M., Vinciguerra, I., Ferrone, A., Riccione, G., Antonia, P., Grilli, A.,
[17]. Felaco, M. and Speranza L. (2011). Licocalchone-C extracted from Glycyrrhiza glabra inhibits Lipopolysaccharide-Interferon-γ inflammation by improving antioxidant conditions and regulating inducible nitric oxide synthase expression. Molecules. 16: 5720-5734.
[18]. Gacche, R., Shaikh, R., Pund, M. and Rupesh, Deshmukh. (2012). Cyclooxygenase inhibitory, cytotoxicity and free radical scavenging activities of selected medicinal plants used in Indian traditional medicine. Pharmacognosy Journal. 3: 57-64.
[19]. Halliwell, B. (2001). Free radicals and other reactive species in disease. Encyclopedia of Life Sciences. Nature Publishing Group. 1-7.
[20]. Hamilton, CA., Miller, WH., Al-Benna, S., Brosnan, MJ, Drummond, RD., McBride, MW and Dominiczak, AF. (2004). Strategies to reduce oxidative stress in cardiovascular disease. Clinical Science. 106: 219–234.
[21]. Hernandez, Y., Sotolongo, J. and Fukata, M. (2011). Toll-like receptor 4 signaling integrates intestinal inflammation with tumorigenesis: Lessons from the murine model of colitis-associated cancer. Cancers. 3: 3104-3113.
[22]. Hong, Y., Chao, W., Chen, M. and Lin B. (2009). Ethyl acetate extracts of alfalfa (Medicago sativa L.) sprouts inhibit lipopolysaccharide-induced inflammation in vitro and in vivo. Journal of Biomedical Science. 16: 64-75.
[23]. Honore P, Donnelly-Roberts D, Namovic M, Chong C, Wade C, Chandran P, et al. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1alphabeta knockout mice. Behav Brain Res. 2009; 204(1):77-81.
[24]. Hsu, D., Chen, S., Chu, P. and Liu, M. (2013). Therapeutic effects of sesame oil on monosodium urate crystal-induced acute inflammatory response in rats. Springer Plus. 2: 659-668.
[25]. Jerca, L., Jerca, O., Mancaş, G., Constantinescu, I. and Lupuşoru, R. (2002). Mechanism of action and biochemical effects of nitric oxide (NO•). The Journal of Preventive Medicine. 10: 35-45.
[26]. Kim, SF., Huri, DA and Snyder, SH. (2005). Inducible nitric oxide synthase binds, Snitrosylates, and activates cyclooxygenase-2 Heyne ex. Roth in rats. International Journal of Comprehensive Pharmacy. 1: 1-3.
[27]. Krishnaraja, AV., Rao, CBM, Sundararaja, D., Sengupta, K. and Trimurtulu, G. (2009). Anti-inflammatory activity of Vitex leucoxylon L. Bark extracts against Freund’s complete adjuvant induced arthritis in Sprague Dawley rats. American Journal of Infectious Diseases. 5: 68-73.
[28]. Kumar, PP., Kumaravel, S. and Lalitha, C. (2010). Screening of antioxidant activity, total phenolics and GCMS study of Vitex negundo. African Journal of Biochemical Research. 47:191-195.
[29]. Kunwar, A. and Priyadarsini, KI. (2011). Free radicals, oxidative stress and importance of antioxidants in human health. Journal of Medical and Allied Sciences. 1: 53-56.
[30]. Lam, ANC., Demasi, M., James, MJ, Husband, AJ. And Walker, C. (2004). Effect of red clover isoflavones on Cox-2 Activity in murine and human monocyte/macrophage cells. Nutrition and Cancer. 49: 89–93.
[31]. Lawlor, G., Doran, PP., MacMathuna, P. and Murray, D. (2010). MYEOV (myeloma overexpressed gene) drives colon cancer cell migration and is regulated by PGE2. Journal of Experimental and Clinical Research. 29: 81.
[32]. Lee, S., Shin, S., Kim, H., Han, S., Kim, K., Kwon, J., Kwak, J., Lee, C., Ha, N., Yim, L. and
[33]. Kim, K. (2011). Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF- Κβ pathways. Journal of Inflammation. 8: 16-24.
[34]. Lin, C., Crawford, DR., Lin, S., Hwang, J., Sebuyira, A, Meng, R., Westfall, JE, Tang, H., Lin, S., Yu, P., Davis, PJ. And Lin, H. (2011). Inducible COX-2 dependent apoptosis in human ovarian cancer cells. Carcinogenesis. 32: 19-26.
[35]. Lobo, V., Patil, A., Phatak, A. and Chandra, N. (2010). Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews. 4: 118–126.
[36]. Marnett, LJ. (2009). Mechanisms of cyclooxygenase-2 inhibition and cardiovascular side effects the plot thickens. Cancer Prevention Research. 2: 288-291.
[37]. Masoko P. and Eloff JN. (2007). Screening of twenty-four South African Combretum and six Terminalia species (Combretaceae) for antioxidant activities. African Journal of Traditional, Complementary and Alternative Medicines. 4: 231-239.
[39]. McDaid, C., Maund, E., Rice, S., Wright, K., Jenkins, B. and Woolacott N. (2010). Paracetamol and selective and non-selective non-steroidal anti- inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: a systematic review. Health Technology Assessment. 14: 1-180.
[40]. Milano, S., Arcoleo, F., Dieli, M., D'Agostino, R., D'Agostino, P., De Nucci, G. and Cillari, E. (1995). Prostaglandin E2 regulates inducible nitric oxide synthase in the murine macrophage cell line J774. Prostaglandins. 49: 105-15.
[41]. Moalem G and Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006; 51(2):240-64.
[42]. Mulabagal, V., Alexander-Lindo, RL., DeWitt, DL and Nair, MG. (2011). Health beneficial phenolic aldehyde in Antigonon leptopus tea. Evidence-based Complementary and Alternative Medicine. 10: 1093-1098.
[43]. Pala, FS., and Gurkan, H. (2008). The role of free radicals in ethiopathogenesis of diseases. Advances in Molecular Biology. 1: 1-9.
[44]. Park, G., Jun, J. and Kim, J. (2013). XH-14, a novel danshen methoxybenzo [b]furan derivative, exhibits anti-inflammatory properties in lipopolysaccharide treated RAW 264.7 cells. Journal of Inflammation. 10: 1.
[46]. Patel, VR., Patel, PR., and Kajal, SS. (2010). Antioxidant activity of some selected medicinal plants in Western region of India. Advances in Biological Research. 4: 23-26.
[47]. Payne, R. (2000). Limitations of NSAIDs for pain management: Toxicity or lack of efficacy. Journal of Pain. 1: 14-18.
[48]. Pegg, RB. (2009). Characterization of the anti-inflammatory properties of Georgia-grown blackberries. (Rubus sp.) 1-5.
[49]. Posadas, I., Rosa, S., Terencio, C., Paya, M. and Alcaraz, J. (2003). Cacospongionolide B suppresses the expression of inflammatory enzymes and tumour necrosis factor-a by inhibiting nuclear factor-kB activation. British Journal of Pharmacology. 138: 1571–1579.
[50]. Ricciotti, E. and Fitzgerald, GA. (2011). Prostaglandins and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 31: 986-1000.
[51]. Sakat, SS., Juvekar, AR. and Gambhire, MNJ. (2010). In vitro antioxidant and anti-inflammatory activity of methanol Oxalis corniculata Linn. International Journal of Pharmacy and Pharmaceutical Sciences. 2: 146-155.
[52]. Sales, KJ., and Jabbour, HN. (2003). Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction. 126: 559-567.
[53]. Rao, AK. (2010). The tale of two COXs. Blood. 115: 921-922.
[54]. Tung, Y., Chua, M., Wang, S. and Chang, S. (2008). Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresource Technology. 99: 3908–3913.
[55]. Turini, ME., and DuBois, RN. (2002). Cyclooxygenase-2: A therapeutic target. Annual Review of Medicine. 53: 35–57.
[56]. Vadivu, R. and Lakshmi, KS. (2011). In vitro and in vivo anti-inflammatory activity of leaves of Symplocos cochinchnensis (Lour) Moore ssp laurina. Bangladesh Journal of Pharmacology. 3: 121-124.
[57]. Wakefield, D. and Kumar, RK. (2001). Inflammation: Chronic. Encyclopedia of Life Sciences. Nature Publishing Group.
[58]. Wang, D. and DuBois, RN. (2006). Prostaglandins and cancer. Gut. 55: 115-122.
[59]. Wei XH, Zang Y, Wu CY, Xu JT, Xin WJ, Liu XG. Peri-sciatic administration of recombinant rat TNF-alpha induces mechanical allodynia via up regulation of TNF-alpha in dorsal root ganglia and in spinal dorsal horn: the role of NF-kappa B pathway. Exp Neurol. 2007; 205(2):471-84.
[60]. Whitehead KJ, Smith CG, Delaney SA, Curnow SJ, Salmon M, Hughes JP, et al. Dynamic regulation of spinal pro-inflammatory cytokine release in rat in vivo following peripheral nerve injury. Brain Behav Immun. 2010; 24(4):569-76.
[61]. Wu, C., Lii, C., Liu, K., Chen, P. and Hseih, S. (2013). Anti-inflammatory activity of Gynura bicolor ether extract through inhibits nuclear factor kappa B activation. Journal of Traditional and Complementary Medicine. 3: 1-7.
[62]. Yan, Y., Li, J., Ouyang, W., Ma Q., Hu, Y., Zhang, D., Ding, J., Qu, Q., Subbaramaiah, K. and Huang, C. (2006). NFAT3 is specifically required for TNF- α-induced cyclooxygenase-2 (COX- 2) expression and transformation of Cl41 cells. Journal of Cell Science. 119: 2985-2995.
[63]. Zhuang, H., Pin, S., Li, X. and Doré, S. (2003). Regulation of heme oxygenase expression by cyclopentenone prostaglandins. Experimental Biology and Medicine. 228: 499-505.
[64]. Zhao, F., Wang, L. and Liu, K. (2009). In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L., through inhibition on iNOS pathway. Journal of Ethnopharmacology. 122: 457–462.
Viewed PDF 2726 63 -
 Phytochemical Analysis and Partial Characterization Caralluma Attenuata Extract by TLCAuthor: A. Chandra MohanDOI: 10.21522./TIJBMS.2016.02.01.Art002
Phytochemical Analysis and Partial Characterization Caralluma Attenuata Extract by TLCAuthor: A. Chandra MohanDOI: 10.21522./TIJBMS.2016.02.01.Art002Phytochemical Analysis and Partial Characterization Caralluma Attenuata Extract by TLC
Abstract:
Medicinal and natural herbal plant products are traditionally used from long time in many countries. The current work was to evaluate the flavonoid rich fraction in Caralluma attenuate and other phytochemical analysis of stem of the plant. Preliminary phytochemical analysis revealed the presence of phytochemicals such as alkaloids, polyphenols, flavonoids and tannin content in methanol extracts of stem then they were determined spectrometrically. The present study provided, a detailed report on the isolation and characterization of Thin Layer Chromatography from stem of Caralluma attenuate. The methanol extract were used for various biological properties and in vivo assays which is used discovering new drugs.
Keywords: Caralluma attenuata, phytochemical screening, medicinal uses and TLC.
Phytochemical Analysis and Partial Characterization Caralluma Attenuata Extract by TLC
References:
[1]. Ajit K.D, Dutta B. K and Sharma G. D (2007) “Medicinal plants used by different tribes of Cachar district, Assam”. Indian Journal Of traditional KnowledgeVol7 (3), pp 446-454.
[2]. Balasubramanian.P, Rajasekaran.A and Prasad.S.N (1997) “Folk Medicine of the Irulas of Coimbatore Forests”. Ancient Science of Life Vol XVI 3, pages 222 – 226.
[3]. Beckman CH. Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol 2000; 57:101-10.
[4]. Brahmachari G. Mother Nature: An inexhaustible source of drugs and lead molecules. In: Brahmachari G, Editor. Chemistry, Biochemistry and Pharmacology. 1 st ed. New Delhi: Narosa Publishing House Pvt. Ltd.; 2009. p. 1-20.
[5]. Buenz E.J, Schnepple D.J and Bauer B.A (2004). Techniques: Bioprospecting historical herbal texts by hunting for new leads in old tomes. Trends PharmacolSci 25: pp.494-498.
[6]. Divya. S., Naveen. K.K., Ramachandran. S and Dhanaraju. M. D. (2011) “Wound Healing and In Vitro Antioxidant Activities of Croton bonplandianum Leaf Extract in Rats”. Global Journal of Pharmacology 5 (3): 159-163.
[7]. Kirti S Prabhu, Richard Lobo and Annie Shirwaikar. Antidiabetic properties of the alcoholic extract of Sphaeranthus indicus in streptozotocin-nicotinamide diabetic rats. Journal of Pharmacy and Pharmacology, 2008; 60: 909-16.
[8]. Kovalev S. V. (2009). Flavonoids from Lotus ucrainicus and I. arvensis. Chemistry of Natural Compounds, Vol. 45, No. 4. Pp. 550-551.
[9]. Lenin B.J and Venkat R.S. (2009). “Traditional Uses of Some Medicinal Plants by tribals of Gangaraju Madugula Mandal of Visakhapatnam District, Andhra Pradesh”. Ethnobotanical Leaflets 13: 388-98.
[10]. Mahadeswara Swamy (2006) Ban Tulsi (Online), Available: http://www.flowersofindia.net/catal og/slides/Ban%20Tulsi.html (25 Jul, 2006).
[11]. Scalbert, A., Manach, C., Morand, C., Remesy, C., Jimenez, L., (2005). Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 45, pp.287-306.
[12]. Spencer JP, Abd El Mohsen MM, Minihane AM, Mathers JC. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr 2008; 99:12-22.
Viewed PDF 2413 43 -
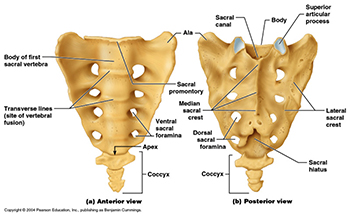 Sacral Hiatus - A Morphometric and Anatomical StudyAuthor: Jyothinath KothapalliDOI: 10.21522./TIJBMS.2016.02.01.Art003
Sacral Hiatus - A Morphometric and Anatomical StudyAuthor: Jyothinath KothapalliDOI: 10.21522./TIJBMS.2016.02.01.Art003Sacral Hiatus - A Morphometric and Anatomical Study
Abstract:
To accurate performance of epidural anaesthesia and analgesia it is important to know the variations of sacral hiatus in dry bone. The present observational study conducted on one hundred dry human sacra to evaluate the anatomical and morphometric variations of sacral hiatus. The most common shape of sacral hiatus was inverted ‘U’ and ‘V’ respectively. Apex of sacral hiatus present at 3rd sacral segment in 78% sacra and base is at fifth sacral segment in 89% sacra. The length of sacral hiatus is between 11-20 mm in majority sacra, anteroposterior diameter is 4-6 mm in 63% sacra and transverse diameter is 11-15 mm in of sacral hiatus. Anatomical variations in sacral hiatus can leads to caudal epidural anaesthesia failure and procedure related complications. Understanding these variations may improve success of caudal epidural anaesthesia and decrease incidence of complications.
Keywords: Sacral hiatus, caudal epidural anaesthesia, Morphometry.
Sacral Hiatus - A Morphometric and Anatomical Study
References:
[1]. Kumar V, Pandey SN, Bajpai RN, Jain PN, Longia GS. Morphometric study of sacral hiatus. J Anat Soc India. 1992; 41:7–13.
[2]. Lanier VS, Mcknight HE, Trotter M. Caudal analgesia: An experimental and anatomical study. American journal of Obstetrics and Gynaecology 1944; 47(5): 633 – 641.
[3]. Letterman GS, Trotter M. Variations of the male sacrum: Their significance in caudal analgesia. Surg Gynecol Obstet. 1944; 78:551–5.
[4]. Manisha B. Sinha, Mrithunjay Rathore, Human Prasad Sinha: A Study of variation of sacral hiatus in dry bone in central Indian region; International J. of Healthcare and Biomedical Research, Volume: 2, Issue: 4, July 2014, Pages 46-52.
[5]. Nagar SK. A study of sacral hiatus in dry human sacra. Journal of Anatomical Society of India 2004; 53(2): 18 - 21.
[6]. Standring S, Newell RLM, Collins P, Healy JC. In the back in; Gray’s Anatomy, The anatomical basis of clinical practice. 40th Edition. ISBN; 978-0-8089-2371-8. SPAIN, CHURCHILL Livingstone Elsevier, 2008; pp; 724-5 2.
[7]. Standring S, Ellis H, Healy JC, Johnson D. Gray's anatomy. 39th ed. Vol. 1431. London: Elsevier Churchill Livingstone; 2005. pp. 749–54.
[8]. Sekiguchi M., Yabuki S., Satoh K., Kikuchi S. (2004) an anatomic study of the sacral hiatus: a basis for successful caudal epidural block. Clin. J. Pain 20: 51–54.
[9]. Trotter M, Letterman GS, Gordon S, Variations of the female Sacrum: their significance in continuous caudal anesthesia. Surg., Gynec. And Obst. 1944; 78:419-424.
[10]. Vinod kumar et al. Morphometrical study of sacral hiatus. Journal of Anatomical society of India 1992; 41(1): 7 – 13.
[11]. Waldman SD caudal epidural nerve block; prone position in – Atlas of interventional Pain Management, 2nd edn. Philadelphia; Saunder 2004; 380-92.
Viewed PDF 20478 149 -
 Study of Onset and Duration of Action of Local Anesthetic Eutectic Mixture in New Zealand White RabbitsAuthor: Vijaykumar SDOI: 10.21522./TIJBMS.2016.02.01.Art004
Study of Onset and Duration of Action of Local Anesthetic Eutectic Mixture in New Zealand White RabbitsAuthor: Vijaykumar SDOI: 10.21522./TIJBMS.2016.02.01.Art004Study of Onset and Duration of Action of Local Anesthetic Eutectic Mixture in New Zealand White Rabbits
Abstract:
The present study was designed to investigate the local anesthetic activity of EMLA cream on intact skin of hind limbs of New Zealand white rabbits by employing a novel method “Pedaling reflex in rabbits”. Five different doses of drug ranging from 0.5 to 2.5 mg was selected and applied to the ventral surface of the hind feet of both limbs. Uniform pressure was applied on anesthetized patches with help of a blunted probe. Six pricks were applied at different points on anesthetized patches and loss of pedaling reflex (leg retraction) followed by application of stimulus was considered as sign of anesthesia. A significant reduction in onset of anesthesia and a dose dependent increase in duration of anesthesia were recorded in the present study. The onset and duration of action for local anesthetic EMLA cream depends on dosage and contact time of drug on intact skin.
Keywords: White rabbit, EMLA cream
Study of Onset and Duration of Action of Local Anesthetic Eutectic Mixture in New Zealand White Rabbits
References:
[1]. Brodin A, Nyqvist-Mayer A, Wadsten T, Forslund B & Broberg F (1984). Phase diagram and aqueous solubility of the lidocaine-prilocaine binary system. Journal of Pharmaceutical Sciences 73, 481-484.
[2]. Ehrenstrom Reiz GME & Reiz SLA (1982). EMLA-a eutectic mixture of local anesthetics for topical anesthesia. Acta Anaesthesiologica Scandinavia (Copenhagen) 26, 596-598.
[3]. Evers H, Von Dardel O, Juhlin L, Ohlsen L & Vinnars E (1985). Dermal effects of composition based on the eutectic mixture of lignocaine and prilocaine (EMLA): studies in volunteers. British Journal of Anesthesia 57, 997-1005.
[4]. Hallen B & Uppfeldt A (1982). Does lidocaine prilocaine cream permit pain free insertion of IV catheters in children? Anesthesiology 57, 340-342.
[5]. Hallen B, Olsson GL & Uppfeldt A (1984). Pain free venipuncture: effect of timing of application of local anesthetic cream. Anesthesia 39, 969-972.
[6]. Hallen B, Carlsson P & Uppfeldt A (1985). Clinical study of lignocaine-prilocaine cream to relieve the pain of venipuncture. British Journal of Anaesthesia 57, 326-328.
[7]. Hopkins CS, Buckley CJ & Bush GH (1988). Pain free infection in infants. Anaesthesia 43, 198-201.
[8]. Juhlin L, Evers H & Broberg F (1980). A lidocaine-prilocaine cream for superficial skin surgery and painful lesions. Acta Dermato-venereologica(Stockholm) 60, 544-546.
[9]. Price HV (1988). Lignocaine-prilocaine cream for lumbar puncture in children. Lancet ii (21), 1174.
[10]. Vogel HG (2002). Local anesthetic activity. Drug discovery and evaluation by HANS Gerhard Vogel 2nd edition, 655.
[11]. Wahlstedt C, Kollberg H, Moller C & Uppfeldt A (1984). Lidocaine-prilocaine cream reduces venipuncture pain. Lancet ii (14), 106.
[12]. Young AC, Shorchall A, Haynes W & Young G (1987). Lignocaine-prilocaine cream for lumbar puncture. Lancet ii (26), 1533.
Viewed PDF 2406 27 -
 Neuroprotective Epigenetic and DNA Damage Repairing Molecular Mechanisms of L-Carnitine and its Congeners against Aging and Age-Related Neurodegenerative DiseasesAuthor: Kumar PonnusamDOI: 10.21522./TIJBMS.2016.02.01.Art005
Neuroprotective Epigenetic and DNA Damage Repairing Molecular Mechanisms of L-Carnitine and its Congeners against Aging and Age-Related Neurodegenerative DiseasesAuthor: Kumar PonnusamDOI: 10.21522./TIJBMS.2016.02.01.Art005Neuroprotective Epigenetic and DNA Damage Repairing Molecular Mechanisms of L-Carnitine and its Congeners against Aging and Age-Related Neurodegenerative Diseases
Abstract:
Aging is n ubiquitous biological phenomena characterized by ever-increasing susceptibility to diseases due to increased oxidative stress (OS) and an ultimate severe non-repairable membrane molecular and mitochondrial damages coupled with energy (ATP) depletion. L-Carnitine (β-hydroxy-γ-triethyl amino butyrate) and its congeners plays an essential role in mitochondrial ATP synthesis while being a powerful anti-inflammatory antioxidant and an organic, non-ionic bi-phasic osmolytes. L-carnitine exerts antimutagenic and genome stabilizing effects by increasing mitochondrial metabolism, anneals DNA-strand breaks and enhances genome stability by modulating histones and DNA-repairing enzymes. Poly (ADP-ribose) polymerase-1 (PARP-1) is an abundant nuclear enzyme and normally functions in DNA damage repair mechanism acts as a double-edged sword, which anneals mild repairable DNA damages, but extensive PARP-1 activation can promote cell death through processes involving energy depletion in severe OS. It has been reported that, severe oxidative stress-mediated extensive non-repairable DNA damage can over-activate PARP-1 and consumes enormous NAD+ and consequently ATP, culminating in cell dysfunction or necrosis by autocatalysis of PARP-1 and caspases. The DNA damage associated with OS known to activate DNA repair proteins, including PARP-1, an important biomarker of brain aging and age-related neurodegenerative diseases. This study delineates the neuroprotective epigenetic and DNA-repairing molecular mechanisms of the iron-chelating anti-inflammatory genome stabilizing antioxidant ergogenic aid L-Carnitine and its congeners against selected degenerative diseases such as Parkinson’s disease (PD), Alzheimer’s disease, Amyotrophic Lateral Sclerosis (ALS) and Multiple sclerosis (MS).
Keywords: Aging, Neurodegenerative diseases, Oxidative stress, DNA damage, Poly (ADP-Ribose) polymerse-1, Caspase, ATP, L-Carnitine, Iron-Chelating Anti-inflammatory Antioxidants.
Neuroprotective Epigenetic and DNA Damage Repairing Molecular Mechanisms of L-Carnitine and its Congeners against Aging and Age-Related Neurodegenerative Diseases
References:
[1]. Abd-Allah AR, Al-Majed AA, Al-Yahya AA, Fouda SI, Al-Shabana OA. L-Carnitine halts apoptosis and myelosuppression induced by carboplatin in rat bone marrow cell cultures (BMC). Arch Toxicol 2005; 79: 406-413.
[2]. Arsuini, A. 1992. Carnitine and its acyl esters as secondary antioxidants? Am. Heart J. 123: 1726–1727.
[3]. Abdul Muneer PM, Alikunju S, Szlachetka AM, Haorah J. Inhibitory effects of alcohol on glucose transport across the blood-brain barrier leads to neurodegeneration: preventive role of acetyl-L-carnitine. Psychopharmacology (Berl). 2011 Apr; 214(3):707-718.
[4]. Ando, S., T. Tadenuma, Y. Tanaka et al. 2001. Enhancement of learning capacity and cholinergic synaptic function by carnitine in aging rats. J. Neurosci. Res. 66: 266–271.
[5]. Arduini, A., S. Dottori, F. Molajoni et al. 1995. Is the carnitine system part of the heart antioxidant network? In The Carnitine System: A New Therapeutical Approach to Cardiovascular Medicine, pp. 169–181. Kluwer. Dordrecht.
[6]. Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 1987; 81: 459-469.
[7]. Aliabadi E, Soleimani Mehranjani M, Borzoei Z, Talaei-Khozani T, Mirkhani H, Tabesh H. Effects of L-carnitine and L-acetyl-carnitine on testicular sperm motility and chromatin quality. Iran J Reprod Med. 2012 Mar; 10(2):77-82.
[8]. Angelucci L, Ramacci MT, Taglialatela G, Hulsebosch C, Morgan B, et al. (1988). Nerve growth factor binding in aged rat central nervous system: effect of acetyl-L-carnitine. J Neurosci Res 20: 491–496.
[9]. Arduini A, Rossi M, Mancineili G, Beigiglio M, Scurti R, Radatti GL, Shohet SB. Effect of L-carnitine and acetyl-L-carnitine on the human erythrocyte membrane stability and deformability. Life Sci 1990; 47:2395-2400.
[10]. Abuzahra, Mona Abd El-Latif; Mustafa, Sherifa Abd-Elsalam. Nitric Oxide and Oxidative Stress Properties of L-Carnitine in Diabetic Hypertensive Rats Biochemical & Histological Study. Middle East Journal of Internal Medicine. Jun2014, Vol. 7 Issue 2, p19-31. 13p.
[11]. Andrieu-Abadie N, Jaffrezou JP, Hatem S, Laurent G, Levade T, and Mercadier JJ. L-Carnitine prevents doxorubicin- induced apoptosis of cardiac myocytes: role of inhibition of ceramide generation. FASEB J 13: 1501–1510, 1999.
[12]. Angelucci L, TRamacci M, Taglialatela G, Hulsebosch C, Morgan B, Werrbach-Perez K, Perez-Polo R. Nerve growth factor binding in aged rat central nervous system: effect of acetyl-L-carnitine. J Neurosci Res. 1988 Aug; 20(4):491-496.
[13]. Beal, MF; Lang, AE; AC, L. Neurodegenerative diseases: neurobiology, pathogenesis, and therapeutics. Cambridge, UK New York: Cambridge University Press; 2005.
[14]. Berni, A., Meschini, R., Filippi, S., Palitti, F., Arnicisb, A., Chessab, L., 2008. L--carnitme enhances resistance to oxidative stress by reducing DNA damage in Ataxia telangiectasia cells. Mut. Res. 650, 165-174.
[15]. Bogdanov M, Brown RH, Matson W, et al. Increased oxidative damage to DNA in ALS patients. Free Radical Biology & Medicine 2000; 29(7):652–658.
[16]. Baxter, A. G. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol 7, 904-912, 2007.
[17]. Bonnefont-Rousselot D, Lacomblez L, Jaudon M, et al. Blood oxidative stress in amyotrophic lateral sclerosis. Journal of the Neurological Sciences 2000; 178(1):57–62.
[18]. Boerrigter Miet, Franceschi C, Arrigoni-Martelli E, J.Y. Wei JY, J. Vijg J. The effect of L-carnitine and acetyl-L-carnitine on the disappearance of DNA single-strand breaks in human peripheral blood lymphocytes. Carcinogenesis. 1993; Oct, 14(10), 2131-36.
[19]. Boerrigter ME, Franceschi C, Arrigoni-Martelli E, Wei JY, Vijg J. The effect of L-carnitine and acetyl-L-carnitine on the disappearance of DNA single-strand breaks in human peripheral blood lymphocytes. Carcinogenesis. 1993 Oct;14(10):2131-6
[20]. Boerrigter, M.E., C. Franceschi, E. Arrigoni-Martelli et al. 1993. The effect of Lcarnitine and acetyl-L-carnitine on the disappearance of DNA single-strand breaks in human peripheral blood lymphocytes. Carcinogenesis 14: 2131-2136.
[21]. Bacon, B.R., R. Neill and C.H. Park, 1986. Iron-induced peroxidative injury to isolated rat hepatic mitochondria. J. Free Radicals Biol. Med. 2: 339–342.
[22]. Baghaei A, Solgi R, Jafari A, Abdolghaffari AH, Golaghaei A, Asghari MH, Baeeri M, Ostad SN, Sharifzadeh M, Abdollahi M.Molecular and biochemical evidence on the protection of cardiomyocytes from phosphine-induced oxidative stress, mitochondrial dysfunction and apoptosis by acetyl-L-carnitine. Environ Toxicol Pharmacol. 2016 Mar; 42:30-37.
[23]. Buyse J, Swennen Q, Niewold TA, Klasing KC, Janssens GPJ, Baumgartner M, et al. Dietary l-carnitine supplementation enhances the lipopolysaccharide-induced acute phase protein response in broiler chickens. Veterinary Immunol Immunopathol 2007; 118: 154159.
[24]. Beal, MF, Lang, AE, AC, L. Neurodegenerative diseases: neurobiology, pathogenesis, and therapeutics. Cambridge, UK New York: Cambridge University Press; 2005.
[25]. Bieber LL, Krahling JB, Clarke PR, Valkner KJ, Tolbert NE. Carnitine acyltransfe rases in rat liver peroxisomes. Arch Biochem Biophys 1981; 211: 599-604.
[26]. Bieber LL. Carnitine. Annu Rev Biochem 1988; 57:261-83.
[27]. Brevetti G, Attisano T, Perna S, et al. Effect of L-carnitine on the reactive hyperemia in patients affected by peripheral vascular disease: a double-blind, crossover study. Angiology. 1989; 40:857–862.
[28]. Bresolin, N., L. Freddo, L. Vergani and C. Angelini. 1982. Carnitine, carnitine acyltransferases, and rat brain function. Exp. Neurol. 78: 285–292.
[29]. Costell, M., J.E. O’Cconnor and S. Grisolia. 1989. Age-dependent decrease of carnitine content in muscle of mice and humans. Biochem. Biophys. Res. Commun. 161: 1135–1143.
[30]. Carta, A., Calvani, M., Bravi, D. and Bhuachalla, S. N. (1993). In Alzheimer’s disease: Amyloid Precursor Proteins, Signal Transduction and Neuronal Transplantation, eds. Wurtman, R. J., Corkin, S., Growdon, J. H. & Nitsch, R. M. (New York Academy of Sciences, New York), Vol. 695, pp. 324-326.
[31]. Chang B, Nishikawa M, Sato E, Utsumi K, Inoue M. L-carnitine inhibits cisplatin-induced injury of the kidney and small intestine. Arch Biochem Biophys 2002; 405:55-64.
[32]. Cress, A. P., Fraker, P. J and Bieber, L. L. Carnitine and acylcarnitine levels of human peripheral blood lymphocytes and mononuclear phagocytes. Biochim Biophys Acta 992, 135-139 (1989).
[33]. Cakir O, Erdem K, Oruc A, Kilinc N, Eren N (2003) Neuroprotective effect of N-acetylcysteine and hypothermia on the spinal cord ischemia-reperfusion injury. Cardiovasc Surg 11: 375–379.
[34]. Calabrese V, Scapagnini G, Ravagna A, et al. Disruption of thiol homeostasis and nitrosative stress in the cerebrospinal fluid of patients with active multiple sclerosis: evidence for a protective role of acetylcarnitine. Neurochem Res 2003; 28:1321-1328.
[35]. Craig J. McMackin, Michael E. Widlansky, Naomi M. Hamburg, Alex L. Huang, Susan Weller, Monika Holbrook, Noyan Gokce, Tory M. Hagen, John F. Keaney, Jr., and Joseph A. Vita. Effect of Combined Treatment with Alpha Lipoic Acid and Acetyl-L-Carnitine on Vascular Function and Blood Pressure in Coronary Artery Disease Patients. J Clin Hypertens (Greenwich). 2007 Apr; 9(4): 249-255.
[36]. Chatzinikolaou, G., Karakasilioti, I., and Garinis, G.A. (2014). DNA damage and innate immunity: links and trade-offs. Trends Immunol. 35, 1–7.
[37]. Chiechio S, Caricasole A, Barletta E, Storto M, Catania MV, Copani A, Vertechy M, Nicolai R, Calvani M, Melchiorri D, Nicoletti F. L-Acetylcarnitine induces analgesia by selectively up-regulating mGlu2 metabotropic glutamate receptors. Mol. Pharmacol. 2002; 61:989-96.
[38]. Costell M, O'Connor JE, Grisolía S. Age-dependent decrease of carnitine content in muscle of mice and humans. Biochem Biophys Res Commun. 1989 Jun 30; 161 (3):1135-43.
[39]. Dobrzyńska I, Szachowicz-Petelska B, Skrzydlewska E, Figaszewski Z. Effect of L-carnitine on liver cell membranes in ethanol-intoxicated rats. Chem Biol Interact 2010; 188: 44-51.
[40]. De Rosa M, Boggia B, Amalfi B, Zarrilli S, Vita A, Colao A, et al. Correlation between seminal carnitine and functional spermatozoal characteristics in men with semen dysfunction of various origins. Drugs R D 2005; 6: 1–9.
[41]. Dayanandan, P.Kumar, T. Kalaiselvi and C. Panneerselvam. Effect of L-Carnitine on blood lipid composition in Atherosclerotic rats. J. Clin. Biochem, Nutr. 17, 81- 87, 1994.
[42]. Deeks SG. Antiretroviral agents: the next generation. AIDS Clin. Care. 1998; May, 10 (5):33-6, 39-40. Review.
[43]. Dolezal, V. and S. Tucek. 1981. Utilization of citrate, acetylcarnitine, acetate, pyruvate, and glucose for the synthesis of acetylcholine in rat brain slices. J. Neurochem. 36: 1323–1330.
[44]. Dayanandan, P.Kumar and C. Panneerselvam.Protective role of L-carnitine on liver and heart lipid peroxidation in atheroscerotic rats. J. Nutr. Biochem, 2001 May; 12(5): 254-257.
[45]. Ew132. D’Amours, D., Desnoyers, S., D’Silva, I., and Poirier, G.G. (1999). Poly (ADPribosyl) action reactions in the regulation of nuclear functions. Biochem. J. 342, 249–268.
[46]. Ewan EE, Hagg T. Intrathecal Acetyl-L-Carnitine Protects Tissue and Improves Function after a Mild Contusive Spinal Cord Injury in Rats. J Neurotrauma. 2016 Feb 1; 33(3):269-77.
[47]. Esposito E, Rotilio D, Di Matteo V, Di Giulio C, Cacchio M, Algeri S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiology of Aging 2002; 23 (5):719-735.
[48]. ERijk, M.C., Launer, L.J., Berger, K., Breteler, M.M., Dartigues, J.F., Baldereschi, M., Fratiglioni, L., Lobo, A., Martinez-Lage, J., Trenkwalder, C and Hofman, A. (2000). Prevalence of Parkinson’s disease in Europe: a collaborative study of populationbased cohorts. Neurology, Vol.54, No.11, pp. S21–S23
[49]. Ford, D. A., Han, X., Horner, C. C and Gross, R. W. Accumulation of unsaturated acylcarnitine molecular species during acute myocardial ischemia: metabolic compartmentalization of products of fatty acyl chain elongation in the acylcarnitine pool. Biochemistry 35, 7903-7909, 1996.
[50]. Folts, J.D., Shug, A.L., Koke, J.R. and Bittar, N. (1978) Am. J.Cardiol. 41, 1209-1214.
[51]. Foster DW. The role of the carnitine system in human metabolism. Ann N Y Acad Sci 2004; 1033: 1-16.
[52]. Ferreira GC, McKenna MC.L-Carnitine and Acetyl-L-carnitine Roles and Neuroprotection in Developing Brain. Neurochem Res. 2017 May 16.
[53]. Ferrante RJ, Browne SE, Shinobu LA, et al. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. Journal of Neurochemistry 1997; 69 (5):2064-2074.
[54]. Fernandes S, Salta S, Summavielle T. Methamphetamine promotes α-tubulin deacetylation in endothelial cells: the protective role of acetyl-l-carnitine. Toxicol Lett. 2015 Apr 16; 234 (2):131-8.
[55]. Furuno T, Kanno T, Arita K, Asami M, Utsumi T, Doi Y, Inoue M, Utsumi K. Roles of long chain fatty acids and carnitine in mitochondrial membrane permeability transition. Biochem Pharmacol 2001; 62: 1037-1046.
[56]. Furuno T, Kanno T, Arita K, Asami M, Utsumi T, Doi Y, Inoue M, Utsumi K. Roles of long chain fatty acids and carnitine in mitochondrial membrane permeability transition. Biochem Pharmacol. 2001 Oct 15; 62(8):1037-46.
[57]. Ford DA. Alterations in myocardial lipid metabolism during myocardial ischemia and reperfusion. Prog Lipid Res2002; 41: 6-26.
[58]. Griendling KK, Fitzgerald GA. Oxidative stress and cardiovascular injury: part II: animal and human studies. Circulation. 2003;108: 2034–2040.
[59]. Gottlieb, R.A., Burleson, K.O., Kloner, R.A., Babior, B.M. and Engler, R.L. (1994) J. Clin. Invest. 94, 1621-1628.
[60]. Gasser P, Martina B, Dubler B. Reaction of capillary blood cell velocity in nailfold capillaries to L-carnitine in patients with vasospastic disease. Drugs Exp Clin Res. 1997; 23: 39–43.
[61]. Gurney ME, Cutting FB, Zhai P, et al. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis [see comments]. Annals of Neurology 1996; 39(2): 147-157.
[62]. Grazi G, Meriggioli M, Donati G. Can the treatment with L-carnitine improve the inflammation in chronic hemodialysis patients? G Ital Nefrol 2004; 21: 204–207.
[63]. Gulcin I. Antioxidant and antiradical activities of L-carnitine. Life Sci 2006; 78: 803-811.
[64]. G. E. Kisby, J. Milne, and C. Sweatt, “Evidence of reduced DNA repair in amyotrophic lateral sclerosis brain tissue,” NeuroReport, vol. 8, no. 6, pp. 1337–1340, 1997.
[65]. G. E. Kisby, J. Milne, and C. Sweatt. “Evidence of reduced DNA repair in amyotrophic lateral sclerosis brain tissue,” NeuroReport, vol. 8, no. 6, pp. 1337-1340, 1997.
[66]. Gadaleta MN, Petruzzella V, Fracasso F, Fernandez-Silva P, Cantatore P. Acety-L-carnitine increases cytochrome oxidase subunit I mRNA content in hypothyroid rat Liver. FEBS, Lett, 1990, 277, 191-193.
[67]. Gelbard, H.A., James, H.J., Sharer, L.R., Perry, S.W., Saito, Y., Kazee, A.M., Blumberg, B.M. and Epstein, L.G. (1995) Neuropathol. Appl. Neurobiol. 21, 208-217.
[68]. Gadaleta MN, Petruzzella M, Renis M, Fracasso F, Antatore P. Reduced mitochondrial DNA transcription in two brain regions of senescent rats: Effect of acetyl-L-carnitine. In: S. Quazliariello, F. Papa, Palmieri and C. Seccombe Edn. Structure, function and biogenesis of energy transfer system. Elsevier, Amsterdam, pp. 135-138.
[69]. Gulcin I (2006). Antioxidant and antiradical activities of L-carnitine. Life Sci 78:803-811.
[70]. Gabryel B, Adamek M, Pudelko A, Malecki A, Trzeciak HI. Piracetam and vinpocetine exert cytoprotective activity and prevent apoptosis of astrocytes in vitro in hypoxia and reoxygenation. Neurotoxicology 2002; 23(1):19-31.
[71]. Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010. ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
[72]. Geremia, E., C. Santoro, D. Baratta et al. 1988. Antioxidant action of acetyl-Lcarnitine: in vitro study. Med. Sci. Res. 16: 699-700.
[73]. Gadaleta, M.N., V. Petruzzella, M. Renis et al. 1990. Reduced transcription of mitochondrial DNA in the senescent rat: tissue dependence and effect of L-carnitine. Eur. J. Biochem. 187: 501-506.
[74]. Hagen, T.M., R.T. Ingersoll, C.M. Wehr et al. 1998. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc. Natl. Acad. Sci. USA 95: 9562–9566.
[75]. Hagen, T.M., J. Liu, J. Lykkesfeldt et al. 2002. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc. Natl. Acad. Sci. USA 99: 1870–1875.
[76]. Hagen TM, Moreau R, Suh JH, Visioli F. Mitochondrial decay in the aging rat heart: evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann. N Y Acad. Sci. 2002; 959, 491-507.
[77]. Haorah J, Floreani NA, Knipe B, Persidsky Y. Stabilization of superoxide dismutase by acetyl-l-carnitine in human brain endothelium during alcohol exposure: novel protective approach. Free Radic Biol Med. 2011 Oct 15; 51(8):1601-9.
[78]. Haleagrahara N and Ponnusamy Kumar. Neuroprotective effect of Centella asiatica extract (CAE) on experimentally induced parkinsonism in aged Sprague-Dawley rats. J Toxicol Sci. 2010; 35 (1):41-47.
[79]. Hagen TM, Liu J, Lykkesfeldt J, et al. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci U S A. 2002; 99: 1870-1875.
[80]. Hagen TM, Ingersoll RT, Lykkesfeldt J, et al. (R)-alpha lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999; 13: 411-418.
[81]. Hagen TM, Ingersoll RT, Wehr CM, et al. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc Natl Acad Sci U S A. 1998; 95: 9562-9566.
[82]. Hagen TM, Moreau R, Suh JH, et al. Mitochondrial decay in the aging rat heart: evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann N Y Acad Sci. 2002; 959: 491-507.
[83]. Hind Abdullah Seed Alzahrani. Protective effect of L-carnitine against acrylamide-induced DNA damage in somatic and germ cells of mice. Saudi J Biol Sci. 2011 Jan; 18(1): 29-36
[84]. Ha, H.C., and Snyder, S.H. (1999). Poly (ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc. Natl. Acad. Sci. USA 96, 13978-13982.
[85]. Hagen, T.M., R.T. Ingersoll, J. Lykkesfeldt et al. 1999. (R)-Alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 13: 411-418.
[86]. Hussein Abdelrazik, M.D, Rakesh Sharma, Reda Mahfouz, and Ashok Agarwal. L-Carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertility and Sterility, 2009 Feb; 91(2):589-96.
[87]. Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs & Aging 2001; 18 (9):685-716.
[88]. Hart AM, Wiberg M, Terenghi G. Pharmacological enhancement of peripheral nerve regeneration in the rat by systemic acetyl-L-carnitine treatment. Neurosci. Lett. 2002; 334: 181-185.
[89]. Hiromi Yano, Eri Oyanagi, Yasuko Kato, Yoshiyuki Samejima, Junzo Sasaki and Kozo Utsumi. L-Carnitine is essential to β-oxidation of quarried fatty acid from mitochondrial membrane by PLA2. Molecular and Cellular Biochemistry, Sep 2010, 342, 1, 95-100.
[90]. Hart AM, Wiberg M, Youle M, Terenghi G. Systemic acetyl-L-carnitine eliminates sensory neuronal loss after peripheral axotomy: a new clinical approach in the management of peripheral nerve trauma. Exp. Brain Res. 2002; 145: 182-189.
[91]. Hart AM, Wilson AD, Montovani C, Smith C, Johnson M, Terenghi G, Youle M. Acetyl-l-carnitine: a pathogenesis based treatment for HIV-associated antiretroviral toxic neuropathy. AIDS. 2004; 18:1549-1560.
[92]. Hagen, T.M., V. Vinarsky, C.M. Wehr and B.N. Ames. 2000. (R)-Alpha-lipoic acid reverses the age-associated increase in susceptibility of hepatocytes to tert-butylhydroperoxide both in vitro and in vivo. Antioxid. Redox Signal. 2: 473–483.
[93]. Hagen TM, Ingersoll RT, Lykkesfeldt J, et al. (R)-alphalipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999; 13: 411-418.
[94]. Horiuchi M, Kobayashi K, Tomomura M, Kuwajima M, Imamura Y, Koizumi T, Nikaido H, Hayakawa J, Saheki T. Carnitine administration to juvenile visceral steatosis mice corrects the suppressed expression of urea cycle enzymes by normalizing their transcription. J Biol Chem. 1992; Mar 15, 267(8), 5032-5035.
[95]. H. Kikuchi, A. Furuta, K. I. Nishioka, S. O. Suzuki, Y. Nakabeppu, and T. Iwaki. “Impairment of mitochondrial DNA repair enzymes against accumulation of 8-oxo-guanine in the spinal motor neurons of amyotrophic lateral sclerosis”. Acta Neuropathologica, vol. 103, no. 4, 408-414, 2002.
[96]. Ishii T1, Shimpo Y, Matsuoka Y, Kinoshita K.Anti-apoptotic effect of acetyl-l-carnitine and I-carnitine in primary cultured neurons. Jpn J Pharmacol. 2000 Jun; 83 (2):119-24.
[97]. Javier Blesa, Ines Trigo-Damas, Anna Quiroga-Varela and Vernice R.Jackson-Lewis. Oxidative stress and Parkinson’s disease. Front Neuroanat. 2015 Jul 8; 9: 91-99.
[98]. Jeffrey Rosenfeld and Amy Ellis. Nutrition and Dietary Supplements in Motor Neuron Disease. Phys Med Rehabil Clin N Am. 2008 Aug; 19(3): 573-589.
[99]. Kidd, P.M. 1999. A review of nutrients and botanicals in the integrative management of cognitive dysfunction. Altern. Med. Rev. 4: 144–161.
[100]. Kalaiselvi, T. and C. Panneerselvam. 1998. Effect of L-carnitine on the status of lipidperoxidation and antioxidants in aging rats. J. Nutr. Biochem. 9: 575–581.
[101]. Kaufmann, S.H., Desnoyers, S., Ottaviano, Y., Davidson, N.E., and Poirier, G.G. (1993). Specific proteolytic cleavage of poly (ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 53, 3976–3985.
[102]. Koudelova J, Mourek J, Drahota Z, Rauchova H (1994). Protective effect of carnitine on lipoperoxide formation in rat brain. Physiol Res 43:387-389.
[103]. Kalaiselvi, T and C. Panneerselvam.1998. Effect of L-carnitine on the status of lipidperoxidation and antioxidants in aging rats. J. Nutr. Biochem. 9: 575–581.
[104]. Kanter M, Topcu-Tarladacalisir Y, Parlar S. Antiapoptotic effect of L-carnitine on testicular irradiation in rats. J Mol Histol 2010; 41: 121-128.
[105]. Kidd PM. Multiple sclerosis, autoimmune inflammatory disease: prospects for its integrative management. Altern Med Rev 2001; 6: 540-566.
[106]. Kalman B, Leist TP. A mitochondrial component of neurodegeneration in multiple sclerosis. Neuromolecular Med 2003; 3:147-158.
[107]. Kuratsune H, Yamaguti K, Lindh G et al (2002). Brain regions involved in fatigue sensation: reduced acetylcarnitine uptake into the brain. Neuroimage 17:1256-1265.
[108]. Kumar Ponnusamy, Sindhu Babulogaiah, Neethu Gopal and Srisharan Manavalan.Acetyl-L-Carnitine and Quercetin as PARP-1 Modulators: Implication on Alzheimer’s Disease. Poster Presntation, The Lancet Neurology Conference Preclinical neurodegenerative disease: towards prevention and early diagnosis Oct 19-21, 2016, Park Plaza Riverbank, London, UK. Kumar Ponnusamy, Sindhu Babulogaiah, Neethu Gopal and Srisharan Manavalan. Impact of Poly (ADP-ribose) Polymerase (PARP- Modulators on the Biomarkers of Alzheimer’s Disease in Aged Mice. The Lancet Neurology Conference Preclinical neurodegenerative disease: towards prevention and early diagnosis Oct 19-21, 2016, Park Plaza Riverbank, London, UK.
[109]. K. Ponnusamy and J.R. Naidu. “Neuroprotective effect of L-carnitine and Centella asiatica extract on 6-hydroxydopamine (6-OHDA)-induced changes in the repair-mechanism and genotoxicity in aged rats”, Parkinsonism and Related Disorders, Vol.18, No.2 (Supplement), 11.12.2011.
[110]. Kumar, P and Jegathambigai, R.N. “Levo-Carnitors as emerging neuroprotective molecular medicines”, Parkinsonism and Related Disorders, Vol.18, No.2 (Supplement), P1, 11.12.2011.
[111]. Kira Y, Nishikawa M, Ochi A, Sato E, Inoue M. L-carnitine suppresses the onset of neuromuscular degeneration and increases the life span of mice with familial amyotrophic lateral sclerosis. Brain Research 2006;1070(1):206-214.
[112]. Kidd PM. A review of nutrients and botanicals in the integrative management of cognitive dysfunction. Altern Med Rev 1999; 4: 144-161.
[113]. Korn, T et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med 13, 423-431 (2007).
[114]. Kornek, B. et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol 157, 267-276, 2000.
[115]. Kocogullari CU, Becit N, Erkut B, Keles MS, Ceviz M, et al. (2008) Prevention of reperfusion injury of the spinal cord in aortic surgery: an experimental study. Surg Today 38: 237–244.
[116]. Liu J, Killilea DW, Ames B. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats’ acetyl-L-carnitine and/or R-lipoic acid. PNAS 2002; 99(4): 1876-1881.
[117]. Liu, J., E. Head, A. Gharib et al. 2002. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-α-lipoic acid. Proc. Natl. Acad. Sci. USA 99: 2356–2361.
[118]. Lee, V. M. and Trojanowsky, J.Q. (2006). Mechanisms of Parkinson’s disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron, Vol.52, No.1, (October 2006), pp. 33–38.
[119]. Langelier, M.-F., Planck, J.L., Roy, S., and Pascal, J.M. (2012). Structural basis for DNA damage-dependent poly (ADP-ribosyl) action by human PARP-1. Science 336, 728–732.
[120]. Liu J, Head E, Kuratsune H, Cotman CW, Ames BN. Comparison of the effects of l-carnitine and acetyl-l-carnitine on carnitine levels, ambulatory activity and oxidative stress biomarkers in the brain of old rats. Ann N Y Acad Sci 2004; 1033: 117-31.
[121]. Liu, J., H. Atamna, H. Kuratsune and B.N. Ames. 2002. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann. N.Y. Acad. Sci. 959: 133–166.
[122]. Lane, T. E. et al. A central role for CD4 (1) T cells and Rantes in virus-induced central nervous system inflammation and demyelination. J Virol 74, 1415-1424, 2000.
[123]. Lu F, Selak M, O’Connor J, et al. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci 2000;177 : 95-103.
[124]. Linnik, M.D., Zobrist, R.H. and Hat¢eld, M.D. (1993) Stroke 24, 2002, 2008.
[125]. Luo, X., B. Reicheter, J. Trines et al. 1999. L-Carnitine attenuates doxorubicin induced lipid peroxidation in rats. J. Free Radicals Biol. Med. 26: 1158–1165.
[126]. Murray WJ, Reed KW, Roche EB. Conformations of carnitine and acetylcarnitine and the relationship to mitochondrial transport of fatty acids. J Theor Biol 1980; 82:559-572.
[127]. Maccari, F., A. Arseni, P. Chiodi et al. 1990. Levels of carnitines in brain and other tissues of rats of different ages: effect of acetyl-L-carnitine administration. Exp.Gerontol. 25: 127-134.
[128]. Maccari, F., Arseni, A., Chiodi, P., Ramacci, M. T. and Angelucci, L. (1990). Exp. Gerontol. 25:127-134.
[129]. Martha C. Mutombaa, Hua Yuana, Mary Konyavkoa, Souichi Adachia, Christopher B. Yokoyamaa, Victoria Esserb, J. Denis McGarryb, Bernard M. Babiora, Roberta A. Gottlieba. Regulation of the activity of caspases by L-carnitine and Palmitoylcarnitine. FEBS Letters 478, (2000), 19-25.
[130]. McEwen ML, Sullivan PG, Rabchevsky AG, Springer JE (2011). Targeting mitochondrial function for the treatment of acute spinal cord injury. Neurotherapeutics 8: 168–179.
[131]. Malek Mahdavi A, Mahdavi R, Kolahi S.Effects of L-Carnitine Supplementation on Serum Inflammatory Factors and Matrix Metalloproteinase Enzymes in Females with Knee Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J Am Coll Nutr. 2016 Sep-Oct; 35(7):597-603.
[132]. Mariano Malaguarnera, Marco Vacante, Teresio Avitabile, Marcella Malaguarnera, Lisa Cammalleri, and Massimo Motta. L-Carnitine supplementation reduces oxidized LDL cholesterol in patients with diabetes. Am J Clin Nutr. 2009 Jan; 89 (1):71-76.
[133]. Mckay Hart, A., M. Wiberg and G. Terenghi. 2002. Pharmacological enhancement of peripheral nerve regeneration in the rat by systemic acetyl-L-carnitine treatment. Neurosci. Lett. 334: 181-185.
[134]. Montgomery, S.A., L.J. Thal and R. Amrein. 2003. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer’s disease. Int. Clin. Psychopharmacol. 18: 61–71.
[135]. Manfridi A, Forloni GL, Arrigoni-Martelli E, Mancia M (1992) Culture of dorsal root ganglion neurons from aged rats: effects of acetyl-L-carnitine and NGF. Int J Dev Neurosci 10: 321–329.
[136]. Mutomba MC, Yuan H, Konyavko M, Adachi S, Yokoyama CB, Esser V, McGarry JD, Babior BM, Gottlieb RA. Regulation of the activity of caspases by L-carnitine and palmitoylcarnitine. FEBS Lett. 2000 Jul 28; 478 (1-2):19-25.
[137]. Matera M, Bellinghieri G, Costantino G, SantoroD, Calvani M, Savica V. History of L-carnitine: Implications for renal disease. J Ren Nutr 2003; 13 : 2-14.
[138]. Mutomba MC, Yuan H, Konyavko M, Adachi S,Yokoyama CB, Esser V, McGarry JD, BabiorBM, Gottlieb RA. Regulation of the activity of caspases by L-carnitine and palmitoylcarnitine, FEBS Lett 2000; 478: 19-25.
[139]. Monti D , Troiano L, Tropea F, Grassilli E, Cossarizza A, Barozzi D, Pelloni MC, Tamassia MG, Bellomo G, Franceschi C. Apoptosis-programmed cell death: a role in the aging process? .Am J Clin Nutr. 1992; Jun, 55(6 Suppl):1208S-14S. Review.
[140]. Moretti S, Alesse E, Di Marzio L, Zazzeroni F, Ruggeri B, Marcellini S, Famularo G, Steinberg SM, Boschini A, Cifoe MG, De Simone C. Effect of L-carnitine on human immunodeficiency virus-1 infection-associated apoptosis: a pilot study. Blood. 1998; May 15, 91(10), 3817-24.
[141]. Mazzio E, Yoon KJ, Karam FA, Mazzio SE, Soliman KF. D-(+)-Acetyl-L-carnitine cytoprotection against 1-methyl-4-phenylpyridinium toxicity in neuroblastoma cells. Biochem Pharmacol 2003; 66:297-306.
[142]. M. Bogdanov, R. H. Brown, W. Matson et al., “Increased oxidative damage to DNA in ALS patients,” Free Radical Biology and Medicine, vol. 29, no. 7, pp. 652-658, 2000.
[143]. Nagaraja Haleagrahara, Cheng Jun Siew and Kumar Ponnusamy. “Effect of quercetin and desferrioxamine on 6-hydrocydopamine (6-OHDA) induced neurotoxicity in striatum of rats. J. Toxicol. Sci., Vol: 38, No: 1, 25-33, 2013.
[144]. Orrell RW, Lane JM, Ross MA. Antioxidant treatment for amyotrophic lateral sclerosis / motor neuron disease. Cochrane Database Syst Rev 2005; 1: CD002829.
[145]. Plioplys AV, Bagherpour S, Kasnicka I. L-Carnitine as a treatment of lethargy in children with chronic neurologic handicaps. Brain Dev 1994; 16:146-149.
[146]. Plioplys AV, Plioplys S. Amantadine and L-carnitine treatment of chronic fatigue syndrome. Neuropsychobiology 1997; 35:16-23.
[147]. Paradies, G., Ruggiero, F. M., Petrosillo, G., Gadaleta, M. N. and Quagliariello, E. (1995) Mech. Ageing Dev. 84, 103-112.
[148]. Paradies, G., Ruggiero, F. M., Gadaleta, M. N. and Quagliariello, E. (1992). Biochim. Biophys. Acta, 1103:324-326.
[149]. Pettegrew JW, Levine J, McClure RJ (2000). Acetyl-L-carnitine physicalchemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer’s disease and geriatric depression. Mol Psychiatry 5: 616-632.
[150]. Paulson, D.J., A.L. Shug and J. Zhao. 1992. Protection of the ischemic diabetic heart by L-propionylcarnitine therapy. Mol. Cell. Biochem. 116: 131–137.
[151]. Paulson DJ, Traxler J, Schmidt M, Noonan J, Shug AL. Protection of the ischaemic myocardium by L-propionylcarnitine: effects on the recovery of cardiac output after ischaemia and reperfusion, carnitine transport, and fatty acid oxidation. Cardiovasc Res. 1986 Jul; 20(7):536-41.
[152]. Piero Ruggenenti, Dario Cattaneo, Giacomina Loriga, Franca Ledda, Nicola Motterlini, Giulia Gherardi, Silvia Orisio, Giuseppe Remuzzi. Ameliorating Hypertension and Insulin Resistance in Subjects at Increased Cardiovascular Risk: Effects of Acetyl-L-Carnitine Therapy. Hypertension. 2009; 54:567-574.
[153]. Paradies, G., F.M. Ruggiero and P. Dinoi. 1992. Decreased activity of the phosphate carrier and modification of lipids in cardiac mitochondria from senescent rats. Int. J. Biochem. 24: 783–787.
[154]. Paradies, G., F.M. Ruggiero, G. Petrosillo et al. 1994. Effect of aging and acetyl-Lcarnitine on the activity of cytochrome oxidase and adenine nucleotide translocase in rat heart mitochondria. FEBS Lett. 350: 213–215.
[155]. Paradies, G., G. Petrosillo, M.N. Gadeleta and F.M. Ruggiro. 1999. The effect of aging and acetyl-L-carnitine on the pyruvate transport and oxidation in rat heart mitochondria. FEBS Lett. 454: 207–209.
[156]. Pillich RT, Scarsella G, Risuleo G. Reduction of apoptosis through the mitochondrial pathway by the administration of acetyl-L-carnitine to mouse fibroblasts in culture. Exp Cell Res 2005; 306: 1-8.
[157]. P.Kumar, R.Sutha, G.Kavitha, K.Suresh Kanna A.Murugesan and S.Sreelatha, International Medical University, Kuala Lumpur, Malaysia, EPHAR 2008 Congress Abstract C002, Manchester, British Pharmacology Society (BPS) UK, 13-17 July 2008, Fundamental & Clinical Pharmacology, Vol 22, Issue s2 (p 47-55), Aug 2008.
[158]. Paradies, G., Ruggiero, F. M., Petrosillo, G., Gadaleta, M. N. and Quagliariello, E. (1994) FEBS Lett. 350, 213–215.
[159]. Qi SN, Zhang ZF, Wang ZY, Yoshida A, Ueda T. L-carnitine inhibits apoptotic DNA fragmentation induced by a new spin-labeled derivative of podophyllotoxin via caspase-3 in Raji cells. Oncol Rep 2006; 15: 119-22.
[160]. Rabchevsky AG, Patel SP, Springer JE (2011). Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol Ther 132: 15–29.
[161]. Rani, P.J. and C. Panneerselvam. 2002. Effect of L-carnitine on brain lipid peroxidation and antioxidant enzymes in old rats. J. Gerontol. Biol. Sci. Med. Sci. 57: B134–B137.
[162]. Rezenik, A.Z., V.E. Kagan, R. Ramsey et al. 1992. Antiradical effects in L-propionyl carnitine protection of the heart against ischemia-reperfusion injury: the possible role of iron chelation. Arch. Biochem. Biophys. 296: 394-401.
[163]. Ruggenenti P, van der Meer IM, Remuzzi G. Oral acetyl-L-carnitine therapy and insulin resistance. Hypertension. 2010 Jun; 55(6):e26.
[164]. Rani, P.J. & C. Panneerselvam. 2002. Effect of L-carnitine on brain lipid peroxidation and antioxidant enzymes in old rats. J. Gerontol. Biol. Sci. Med. Sci. 57: B134–B137.
[165]. Rahman A, Ustundag B, Burma O, Ozercan IH, Erol FS (2001). Neuroprotective effect of regional carnitine on spinal cord ischemia–reperfusion injury. Eur J Cardiothorac Surg 20: 65–70.
[166]. Rani PJ, Panneerselvam C (2002). Effect of L-carnitine on brain lipid peroxidation and antioxidant enzymes in old rats. J Gerontol A Biol Sci Med Sci 57:B134-B137.
[167]. R. J. Ferrante, S. E. Browne, L. A. Shinobu et al., “Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis,” Journal of Neurochemistry, vol. 69, no. 5, pp. 2064-2074, 1997.
[168]. Reznick AZ, Kagan VE, Ramsey R, Tsuchiya M, Khwaja S, Serbinova EA et al (1992). Antiradical effects in propionyl carnitine protection of the heart against ischemia-reperfusion injury: the possible role of iron chelation. Arch Biochem Biophys 296:394-401.
[169]. Rani PJ and Panneerselvam C (2002). Effect of L-carnitine on brain lipid peroxidation and antioxidant enzymes in old rats. J Gerontol A Biol Sci Med Sci 57:B134-B137.
[170]. Saunders CD. Parkinson’s disease: a new hope. Boston MA: Harvard Health Publication; 2000.
[171]. Siliprandi N, Siliprandi D, Ciman M. Stimulation of oxidation of mitochondrial fatty acids and of acetate by acetylcarnitine. Biochem J 1965; 96: 777-780.
[172]. Solarska K, Lewińska A, Karowicz-Bilińska A, Bartosz G.The antioxidant properties of carnitine in vitro. Cell Mol Biol Lett 2010; 15: 90-97.
[173]. Savica V, Santoro D, Mazzaglia G et al. L-carnitine infusions may suppress serum C-reactive protein and improve nutritional status in maintenance hemodialysis patients. J Ren Nutr 2005; 15: 225–230
[174]. Schinetti, M.L., D. Rossini, R. Greco and A. Bertelli. 1987. Protective action of acetylcarnitine on NADPH-induced lipid peroxidation of cardiac microsomes. Drugs Exp. Clin. Res. 13: 509–515.
[175]. Spagnoli, A., Lucca, U., Menasce, G., Bandera, L., Cizza, G., Forloni, G., Tettamanti, M., Frattura, L., Tiraboschi, P. and Comelli, M. (1991) Neurology 41, 1726-1732.
[176]. Salsoso R, Guzmán-Gutiérrez E, Arroyo P, Salomón C, Zambrano S, Ruiz-Armenta MV, Blanca AJ, Pardo F, Leiva A, Mate A, Sobrevia L, Vázquez CM. Reduced L-Carnitine Transport in Aortic Endothelial Cells from Spontaneously Hypertensive Rats. PLoS One. 2014 Feb 28; 9(2):e90339.
[177]. Sharman, E.H., N.D. Vaziri, Z. Ni et al. 2002. Reversal of biochemical and behavioral parameters of brain aging by melatonin and acetyl L-carnitine. Brain Res. 957: 223–230.
[178]. Scafidi S, Racz J, Hazelton J, McKenna MC, Fiskum G. Neuroprotection by acetyl-L-carnitine after traumatic injury to the immature rat brain. Dev Neurosci. 2010; 32 (5-6):480-7.
[179]. Samejima, K., and Earnshaw, W.C. (2005). Trashing the genome: the role of nucleases during apoptosis. Nat. Rev. Mol. Cell Biol. 6, 677–688.
[180]. Savitha S, Panneerselvam C. Carnitine and lipoic acid alleviates protein oxidation in heart mitochondria during aging process. Biogerontology 2006; 7: 101-109.
[181]. Singh RP, Sharad SKS. Free Radicals and Oxidative Stress in Neurodegenerative Diseases: Relevance of Dietary Antioxidants. Journal of the Indian Academy of Clinical Medicine 2004; 5:218-225.
[182]. Sleem M, Taye A, El-Moselhy MA, Mangoura SA. Combination therapy with losartan and L-carnitine protects against endothelial dysfunction of streptozotocin-induced diabetic rats. Eur J Pharmacol. 2014 Dec 5; 744:10-17.
[183]. Shug AL, Schmidt MJ, Golden GT, et al. The distribution and role of carnitine in the mammalian brain. Life Sci 1982; 31 (25):2869-2874.
[184]. Swerdlow RH, Golbe LI, Parks JK, et al. Mitochondrial dysfunction in cybrid lines expressing mitochondrial genes from patients with progressive supranuclear palsy. J Neurochem 2000; 75: 1681-1684.
[185]. Sener G, Eksioglu-Demiralp E, Cetiner M, Ercan F, Sirvanci S, Gedik N, et al. L-Carnitine ameliorates methotrexate-induced oxidative organ injury and inhibits leukocyte death. Cell Biol Toxicol 2006; 22: 47-60.
[186]. Teichert J, Kern J, Tritschler HJ, et al. Investigations on the pharmacokinetics of alpha-lipoic acid in healthy volunteers. Int J Clin Pharmacol Ther. 1998; 36:625–628.
[187]. Tamilselvan J, Jayaraman G, Sivarajan K, Panneerselvam C. Age-dependent upregulation of p53 and cytochrome c release and susceptibility to apoptosis in skeletal muscle fiber of aged rats: role of carnitine and lipoic acid. Free Radic Biol Med. 2007 Dec 15; 43(12):1656-69.
[188]. Tesco, G., S. Latorraca, P. Piersanti et al. 1992. Protection from oxygen radical damage in human diploid fibroblasts by acetyl-L-carnitine. Dementia 3: 58–60.
[189]. Thomsen, J.H., Shug, A.L., Yap, V.U., Patel, A.K., Karras, T.J. and DeFelice, S.L. (1979) Am. J. Cardiol. 43, 300-306.
[190]. Taglialatela, G., A. Caprioli, A. Giuliani and O. Ghirardi. 1996. Spatial memory and NGF levels in aged rats: natural variability and effects of acetyl-L-carnitine treatment. Exp. Gerontol. 31: 577–587.
[191]. Taglialatela G, Angelucci L, Ramacci MT, Werrbach-Perez K, Jackson GR, et al. (1991) Acetyl-L-carnitine enhances the response of PC12 cells to nerve growth factor. Brain Res Dev Brain Res 59: 221–230.
[192]. Takada G. Komatsu K, Goto R. Carnitine deficiency in lysinuric protein intolerance: lysine-sparing effect of carnitine. Tohoku J Exp Med.1987; Dec, 153 (4), 331-334.
[193]. Tomassini V, Pozzilli C, Onesti E, et al. Comparison of the effects of acetyl L-carnitine and amantadine for the treatment of fatigue in multiple sclerosis: results of a pilot, randomised, doubleblind, crossover trial. J Neurol Sci 2004; 218:103-108.
[194]. Tomassini V, Pozzilli C, Onesti E et al (2004). Comparison of the effects of acetyl-L-carnitine and amantadine for the treatment of fatigue in multiple sclerosis: results of a pilot, randomised, double-blind, crossover trial. J Neurol Sci 218:103-108.
[195]. The Canadian MS Research Group. A randomized controlled trial of amantadine in fatigue associated with multiple sclerosis. Can J Neurol Sci 1987; 14(3):273-278.
[196]. Villa, R. F., Turpeenoja, L., Benzi, G. and Giuffrida Stella, A. M. (1998). Neurochem. Res. 10, 909–916.
[197]. Vernez L, Dickenmann M, Steiger J, Wenk M, Krähenbühl S. Effect of L-carnitine on the kinetics of carnitine, acylcarnitines and butyrobetaine in long-term haemodialysis. Nephrol Dial Transplant. 2006 Feb; 21(2):450-458.
[198]. Villa, R.F., L. Turpeenoja, G. Benzi & A.M. Giuffrida Stella. 1998. Action of L-acetylcarnitine on age-dependent modifications of mitochondrial membrane proteins from rat cerebellum. Neurochem. Res. 10: 909-916.
[199]. Vescovo G, Ravara B, Gobbo V, Sandri M,Angelini A, Della Barbera M, Dona M, Peluso G,Calvani M, Mosconi L, Dalla Libera L. L-carnitine: Apotential treatment for blocking apoptosis and preventing skeletal musclemyopathy in heart failure. Am J Physiol-Cell physiol 2002; 283: C802-C810.
[200]. Vanella, A., Russo, A., Acquaviva, R., Campisi, A., Di Giacomo, C., Sorrenti, V. and Barcellona, M.L. (2000). L-propionylcarnitine as superoxide scavenger, antioxidant and DNA cleavage protector. Cell Biol. Toxicol. , 16 (2): 99-104.
[201]. Vielhaber S, Kaufmann J, Kanowski M, et al. Effect of creatine supplementation on metabolite levels in ALS motor cortices. Exp Neurol 2001; 172: 377-382.
[202]. Vyshkina T, Banisor I, Shugart YY, et al. Genetic variants of complex I in multiple sclerosis. J Neurol Sci 2005; 228:55-64.
[203]. Wilson AD, Hart A, Brannstrom T, Wiberg M, Terenghi G. Primary sensory neuronal rescue with systemic acetyl-Lcarnitine following peripheral axotomy. A dose-response analysis. Br. J. Plast. Surg. 2003; 56: 732-739.
[204]. Waber, L.J., D. Valle, C. Neill et al. 1982. Carnitine deficiency presenting as familial cardiomyopathy: a treatable defect in carnitine transport. J. Pediatr. 101: 700–705.
[205]. Yuji Ueno, Masato Koike, Yoshiaki Shimada, Hideki Shimura, Kenichiro Hira, Ryota Tanaka, Yasuo Uchiyama, Nobutaka Hattori, and Takao Urabe. L-carnitine enhances axonal plasticity and improves white-matter lesions after chronic hypoperfusion in rat brain. J Cereb Blood Flow Metab. 2015 Mar; 35(3): 382-391.
[206]. Yamada, K. A., Kanter, E. M and Newatia, A. Long-chain acylcarnitine induces Ca2+ efflux from the sarcoplasmic reticulum. J Cardiovasc Pharmacol 36, 14-21, (2000).
[207]. Y. Ihara, K. Nobukuni, H. Takata, and T. Hayabara. “Oxidative stress and metal content in blood and cerebrospinal fluid of amyotrophic lateral sclerosis patients with and without a Cu, Zn-superoxide dismutase mutation,” Neurological Research, vol. 27, no. 1, pp. 105-108, 2005.
[208]. Yongkang Dong, , Ling Zhang, Wenjiao Zhu, Hong Chu, Wei Fan, Liming Wu, Jianwei Shao, Weiliang Ding, Tieliang Ma. Modulation of age-associated oxidative DNA damage in rat brain cerebral cortex, striatum and hippocampus by l-carnitine. Experimental Gerontology, 40, 3, 2005, 129-135
[209]. Yongkang Dong, Ling Zhang, Wenjiao Zhu, Hong Chu, Wei Fan, Liming Wu, Jianwei Shao, Weiliang Ding, Tieliang Ma. Effect of L-carnitine on DNA damage and oxidative stress in maintenance hemodialysis patients with hepatitis C virus infection in East China. Int J Clin Exp Med 2016; 9 (3):6100-6106.
[210]. White HL, Scates PW. Acetyl-L-carnitine as a precursor of acetylcholine. Neurochem Res1990; 15: 597-601.
[211]. Yasui, F., S. Matsugo, M. Ishibashi et al. 2002. Effects of chronic acetyl-L-carnitine treatment on brain lipid hydroperoxide level and passive avoidance learning in senescence-accelerated mice. Neurosci. Lett. 334: 177–180.
[212]. Yeun JY, Levine RA, Mantadilok V et al. C-reactive protein predicts all cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2000; 35: 469-476
[213]. Yano T, Itoh Y, Yamada M, Egashira N, Oishi R. Combined treatment with L-carnitine and a pan-caspase inhibitor effectively reverses amiodarone-induced injury in cultured human lung epithelial cells. Apoptosis. 2008 Apr;13(4):543-52.
[214]. Zhou X, Liu F, Zhai S. Effect of L-carnitine and/or L-acetylcarnitine in nutrition treatment for male infertility: a systematic review. Asia Pac J Clin Nutr 2007; 16 Suppl 1: 383-90.
[215]. Zhang ZY, Fan ZK, Cao Y, Jia ZQ, Li G, Zhi XD, Yu DS, Lv G. Acetyl-L-carnitine ameliorates mitochondrial damage and apoptosis following spinal cord injury in rats. Neurosci Lett. 2015 Sep 14; 604: 18-23.
[216]. Zanelli SA, Solenski NJ, Rosenthal RE, Fiskum G. Mechanisms of ischemic neuroprotection by Acetyl-L-carnitine. Ann N Y Acad Sci 2005; 1053: 153-161.
Viewed PDF 2745 49 -
 Antifungal, Contraceptive, Anti-Cancer, Mosquito Repellent Properties of Azadirachta Indica: A reviewAuthor: Niharika AnandDOI: 10.21522./TIJBMS.2016.02.01.Art006
Antifungal, Contraceptive, Anti-Cancer, Mosquito Repellent Properties of Azadirachta Indica: A reviewAuthor: Niharika AnandDOI: 10.21522./TIJBMS.2016.02.01.Art006Antifungal, Contraceptive, Anti-Cancer, Mosquito Repellent Properties of Azadirachta Indica: A review
Abstract:
Azadirachta indica, or more commonly known as Neem, is noted for a variety of medicinal properties. Large numbers of unique phytochemical constituents have been purified from this plant that are being used effectively as anti-fungal, anti-bacterial and anti-inflammatory agents. Neem leaf is an essential ingredient of many Ayurveda medicines. Gedunin and nimbidol are found in the leaves of neem, are shown powerful antifungal activity in various fungal infections in humans. Traditional forms of medicine practiced for centuries in Africa and Asia has been reported that, the leaf extract of Azardica indica possess wound healing activity when applied over external wound and it also taken internally to boost immunity and improve overall health. Several parts of this plant are used in many ailments such as intestinal pain, diabetics, fever, hepatitis, etc. Although the effects of neem are widely known and appreciated, thus far steps haven’t been taken to study its individual properties, and prove through research the extent of its prowess. This review gives a bird’s eye view mainly on the some of the biological activity of the neem includes antifungal, contraceptive and anti-cancer and mosquito repellent properties.
Keywords: Azardica indica, antifungal, contraceptive, anti-cancer, P.ovale, mosquito repellent.
Antifungal, Contraceptive, Anti-Cancer, Mosquito Repellent Properties of Azadirachta Indica: A review
References:
[1]. Akin M., (2010) Neem (Azadirachta indica) Natural Standard, www.natural standard.com.
[2]. Dua, Virendra K.; Pandey, Akhilesh C.; Raghavendra, Kamaraju; Gupta, Ashish; Sharma, Trilochan; Dash, Aditya P. (2009) Larvicidal activity of neem oil (Azadirachta indica) formulation against mosquitoes Malaria Journal.
[3]. Dube S, Tripathi S. (1987) Toxicity of some plants against dermatophytes. National Academy of Sciences, India, Science Letters, 10(2): 45-48.
[4]. Farahna, M., S. Bedri, S. Khalid, M. Idris, C.R. Pillai and E.A. Khalil (2010). Anti-plasmodial effects of Azadirachta indica in experimental cerebral malaria: Apoptosis of cerebellar Purkinje cells of mice as a marker. N. Am. J. Med. Sci., 2: 518-525.
[5]. Fisch I. R., and Frank J. (1977). Oral contraceptives and blood pressure. The Journal of the American Medical Association, 237, 2499–2503.
[6]. Houghton PJ. (1995). The role of plants in traditional medicine and current therapy. J Alter Complement Med, 1:131–43.
[7]. Kabeh JD and M.G.D.S.S. Jalingo (2007) Mini review exploiting neem for improved life / Int J agribiol; vol 9, no.3.
[8]. Kemmeren J. M., Tanis B. C., van den Bosch M. A. A. J., Bollen E. L. E. M., Helmerhorst F. M., van der Graaf Y. and Algra A. (2002). Risk of arterial thrombosis in relation to oral contraceptives (RATIO) study: Oral contraceptives and the risk of ischemic stroke. Stroke, 33, 1202–1208.
[9]. Khillare, B., Shrivastav T.G et al.(2003) Spermicidal activity of Azadirachta indica (neem) leaf extract Contraception Journal , Volume 68 , Issue 3 , 225 - 229
[10]. Mahmoud, D. A., Hassanein, N. M., Youssef, K. A., & Abou Zeid, M. A. (2011). Antifungal activity of different neem leaf extracts and the nimonol against some important human pathogens. Brazilian Journal of Microbiology, 42(3), 1007–1016.
[11]. Moreno V., Bosch F. X., Muñoz N., Meijer C. J. L. M., Shah K. V. and Walboomers J. M. M., . (2002). Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: The IARC multicentric case-control study. The Lancet, 359, 1085–1092.
[12]. Nagpal BN, Srivastava A, Valecha N, Sharma VP, (2001). Repellent action of neem cream against Anopheles culicifacies and Culex quinquefasciatus. Curr Sci; 80: 1270-1.
[13]. Natarajan V, Pushkala S, Karuppiah VP, Prasad PV. (2002) Anti dermatophytic activity of Azardirachta indica (neem) by invitro study. Indian J Pathol Microbiol. Jul; 45(3):311-3.
[14]. Niharika Anand, Arulsamy Anand, Johnson M. Aquicio. (2010) Antifungal properties of neem (azardirachta indica) leaves extract to treat hair dandruff E-International Scientific Research Journal ISSN: 2094-1749 Volume: 2 Issue: 3.
[15]. Okemo PO, Mwatha WE, Chabrab SC, Fabryc W. (2001). The kill kinetics of Azadirachtaindica A. juss. (Meliacae) extracts on Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans. Afr J Sci Technol, 2:113-118
[16]. Patel EK., Gupta A., and Oswai RJ. (2012) A Review On: Mosquito Repellent Method. IJPCBS, 2(3), 310-317
[17]. Patel SM, Nagulapalli Venkata KC, Bhattacharyya P, Sethi G, Bishayee A. (2016) Potential of neem (Azadirachta indica L.) for prevention and treatment of oncologic diseases. Epubmed 2016 Mar 24.
[18]. Paul R1, Prasad M, Sah NK (April 2005) Anticancer biology of Azadirachta indica L (neem): a mini review Current Medicinal Chemistry - Anti-Cancer Agents 5(2):149-6.
[19]. Ping-Hsein, Chi-Weilee, Jia-Ying Chou, Murugan M, Bor-Jinn Shieh, Hueih-Mir Chen.(2007) Antifungal activity of crude extracts and essential oil of Moringa oleifera L. Bioresource Technology, 98: 232-236.
[20]. Sharma V N, Saxena K P. (1959) Spermicidal action of sodium nimbinate Indian journal of medical research, 47, 322-324.
[21]. Subapriya R1, Nagini S. (2005) Medicinal properties of neem leaves: a review. Current Medicinal Chemistry Anticancer Agents. Mar; 5(2):149-6.
[22]. Wong B, Klei B, Kozel T. (1994) Immunologic approaches and metabolite detection. The second NIAID Workshop in Medical Mycology, University of Arizona, Northern Arizona University, Flagstaff, AZ, June 8-11.
Viewed PDF 2867 67

