Prevalence of Pfhrp2 and Pfhrp3 Gene Deletions in Plasmodium Falciparum Isolates and their Performance of Hrp2 Based Malaria Rapid Diagnostic Tests in Three Districts of Ghana
Abstract:
Malaria rapid diagnostic tests (MRDTs)
are important for malaria disease management. However, the performance of the RDTs
is affected when the targeted antigens in the parasite have a variation or are altogether
absent. The most common parasite target antigen in RDTs, Plasmodium falciparum histidine-rich
protein 2 (HRP2), has been reported to be absent in some P. falciparum parasites.
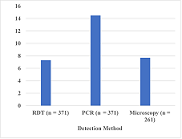
371 patient samples, from Akuapem North (58.5%), Atiwa East (21.3%), and from New
Juaben (20.2%), were used in the study. PCR provided the highest number, 14.8% (55/371),
of positive detections for falciparum infections. Microscopy detected parasites
in 20/261 (7.7%) samples, and the minimum parasite density by microscopy was 430
parasites/µL. Out of the 371 samples, 27 (7.3%) were positive by RDT. The highest
RDT positivity rate, 13.3% (10/75), was observed at New Juaben. False-negative RDT
results were obtained in 43/55 (78.2%) of the negative branded RDT kits. Only two
microscopies positive sample were RDT positive. Using 18SrDNA PCR, 55 (14.8%) samples
were positive for P. falciparum. In Akuapem North, 79.2 % (19/24) of the PCR positive
samples had P. falciparum parasites that lacked exon 2 of PFHRP2. An overall RDT
positivity rate of 7.3% (27/371) and false-negative rate of 78.2% (43/55) were observed
for the study sites. Plasmodium falciparum parasite populations with deletions of
the PFHRP2 and PFHRP3 genes are present in Ghana. There is an urgent need to
investigate the prevalence and geographic distribution of these parasites.
References:
[1] WHO. (2016b). World
Malaria Report, 2016. Retrieved from http://www.who.int/malaria/publications/world-malariareport-2016/report/en/.
[2] WHO. (2018). World
Malaria Report 2018. Retrieved from Geneva: https://www.who.int/malaria/publications/world-malaria-report-2018/en/.
[3] WHO. (2011). Report
on the regional workshop on strengthening quality management systems for parasitological
diagnosis of malaria. Retrieved from http://applications.emro.who.int/docs/IC_Meet_Rep_2012_EN_14512.pdf.
[4] Abba, K., Kirkham,
A. J., Olliaro, P. L., Deeks, J. J., Donegan, S., Garner, P., & Takwoingi, Y.
(2014). Rapid diagnostic tests for diagnosing uncomplicated non-falciparum or Plasmodium
vivax malaria in endemic countries. Cochrane Database Syst Rev (12), Cd011431. doi:10.1002/14651858.cd011431.
[5] Kumar, N., Singh, J.
P., Pande, V., Mishra, N., Srivastava, B., Kapoor, R., Anvikar, A. R. (2012). Genetic
variation in histidine-rich proteins among Indian Plasmodium falciparum population:
a possible cause of variable sensitivity of malaria rapid diagnostic tests. Malar
J, 11, 298. doi:10.1186/1475-2875-11-298.
[6] Lee, N., Gatton, M.
L., Pelecanos, A., Bubb, M., Gonzalez, I., Bell, D., McCarthy, J. S. (2012). Identification
of optimal epitopes for Plasmodium falciparum rapid diagnostic tests that target
histidine-rich proteins 2 and 3. J Clin Microbiol, 50(4), 1397-1405. doi:10.1128/jcm.06533-11.
[7] WHO. (2015b). WHO-FIND
malaria RDT evaluation programme: product testing round 5. Retrieved from http://www.who.int/malaria/mpac/mpac-sept2014-round5-
product- testing-presentation.pdf.
[8] Maltha, J., Guiraud,
I., Lompo, P., Kabore, B., Gillet, P., Van Geet, C., Jacobs, J. (2014). Accuracy
of PFHRP2 versus Pf-pLDH antigen detection by malaria rapid diagnostic tests in
hospitalized children in a seasonal hyperendemic malaria transmission area in Burkina
Faso. Malar J, 13, 20. doi:10.1186/1475-2875-13- 20.
[9]
Mouatcho,
J. C., & Goldring, J. P. (2013). Malaria rapid diagnostic tests: challenges
and prospects. (1473-5644 (Electronic)).
[10] WHO. (2017b). Malaria
rapid diagnostic test performance: results of WHO product testing of malaria RDTs:
round 7 (2015-2016). Retrieved from http://www.who.int/malaria/publications/atoz/978924151268/en/.
[11] http://www.easternregion.gov.gh/index.php/profile/.
[12] Gupta, H., Matambisso,
G., Galatas, B., Cisteró, P., Nhamussua, L., Simone, W., Mayor, A. (2017). Molecular
surveillance of PFHRP2 and PFHRP3 deletions in Plasmodium falciparum isolates from
Mozambique. Malar J, 16. doi:10.1186/s12936-017-2061-z.
[13] WHO. (2017a). False-negative
RDT results and implications of new reports of P. falciparum histidine-rich protein
2/3 gene deletions. Retrieved from https://www.who.int/docs/default-source/documents/publications/gmp/false-negative-rdt-results.pdf?sfvrsn=ec917b72_2.
[14] Baiden, F., Webster,
J., Tivura, M., Delimini, R., Berko, Y., Amenga-Etego, S., Chandramohan, D. (2012).
Accuracy of rapid tests for malaria and treatment outcomes for malaria and non-malaria
cases among under-five children in rural Ghana. PLoS One, 7(4), e34073. doi:
10.1371/journal.pone.0034073.
[15] Nkrumah, B., Acquah,
S. E., Ibrahim, L., May, J., Brattig, N., Tannich, E., Huenger, F. (2011). Comparative
evaluation of two rapid field tests for malaria diagnosis: Partec Rapid Malaria
Test(R) and Binax Now(R) Malaria Rapid Diagnostic Test. BMC Infect Dis, 11, 143.
doi:10.1186/1471-2334-11-143.
[16] Bisoffi, Z., Sirima,
S. B., Menten, J., Pattaro, C., Angheben, A., Gobbi, F., Van den Ende, J. (2010).
Accuracy of a rapid diagnostic test on the diagnosis of malaria infection and malaria-attributable
fever during low and high transmission season in Burkina Faso. Malar J, 9, 192.
doi:10.1186/1475-2875-9-192.
[17] Rakotonirina, H., Barnadas,
C., Raherijafy, R., Andrianantenaina, H., Ratsimbasoa, A., Randrianasolo, L., Menard,
D. (2008). Accuracy and reliability of malaria diagnostic techniques for guiding
febrile outpatient treatment in malaria-endemic countries. Am J Trop Med Hyg, 78(2),
217-221.
[18] Amoah, L. E., Abankwa,
J., & Oppong, A. (2016). Plasmodium falciparum histidine-rich protein-2 diversity
and the implications for PFHRP 2: based malaria rapid diagnostic tests in Ghana.
Malar J, 15, 101. doi:10.1186/s12936-016-1159-z.
[19] Berzosa, P., de Lucio,
A., Romay-Barja, M., Herrador, Z., Gonzalez, V., Garcia, L.,Benito, A. (2018). Comparison
of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria
parasites in representative samples from Equatorial Guinea. Malar J, 17(1), 333.
doi:10.1186/s12936-018-2481-4.
[20] Gatti, S., Gramegna,
M., Bisoffi, Z., Raglio, A., Gulletta, M., Klersy, C., Scaglia, M. (2007). A comparison
of three diagnostic techniques for malaria: a rapid diagnostic test (NOW Malaria),
PCR and microscopy. Ann Trop Med Parasitol, 101(3), 195-204. doi:10.1179/136485907x156997.
[21] Tham, J. M., Lee, S.
H., Tan, T. M., Ting, R. C., & Kara, U. A. (1999). Detection and species determination
of malaria parasites by PCR: comparison with microscopy and with Parasite-F and
ICT malaria Pf tests in a clinical environment. J Clin Microbiol, 37(5), 1269-1273.
[22] Gardner, M. J., Hall,
N., Fung, E., White, O., Berriman, M., Hyman, R. W., Barrell, B. (2002). Genome
sequence of the human malaria parasite Plasmodium falciparum. Nature, 419(6906),
498-511. doi:10.1038/nature01097.
[23] Gamboa, D., Ho, M.
F., Bendezu, J., Torres, K., Chiodini, P. L., Barnwell, J. W., Cheng, Q. (2010).
A large proportion of P. falciparum isolates in the Amazon region of Peru lack PFHRP2
and PFHRP3: implications for malaria rapid diagnostic tests. PLoS One, 5(1), e8091.
doi: 10.1371/journal.pone.0008091.
[24] Abdallah, J. F., Okoth,
S. A., Fontecha, G. A., Torres, R. E., Banegas, E. I., Matute, M. L., Udhayakumar,
V. (2015). Prevalence of PFHRP2 and PFHRP3 gene deletions in Puerto Lempira, Honduras.
Malar J, 14, 19. doi:10.1186/s12936-014-0537-7.
[25] Akinyi, S., Hayden,
T., Gamboa, D., Torres, K., Bendezu, J., Abdallah, J. F., Udhayakumar, V. (2013).
Multiple genetic origins of histidine-rich protein 2 gene deletion in Plasmodium
falciparum parasites from Peru. Sci Rep, 3, 2797. doi:10.1038/srep02797.
[26] Kyabayinze, D. J.,
Zongo, I., Cunningham, J., Gatton, M., Angutoko, P., Ategeka, J., Bell, D. (2016).
HRP2 and pLDH-Based Rapid Diagnostic Tests, Expert Microscopy, and PCR for Detection
of Malaria Infection during Pregnancy and at Delivery in Areas of Varied Transmission:
A Prospective Cohort Study in Burkina Faso and Uganda. PLoS One, 11(7), e0156954.
doi: 10.1371/journal.pone.0156954.

