Anti-inflammatory Effect of Salvia Miltiorrhiza is Mediated via IL-6, JAK, and STAT Pathway in a Dysfunctional Vascular Endothelial Cell
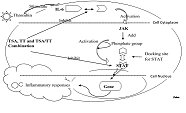
Abstract:
The vascular complication of diabetes
mellitus is a problem for the patient, and the ability to cope with the disease
and the associated inflammation is a critical aspect of diabetes. Cytokines-induced
inflammation in vascular endothelial cells (VECs) plays an active role in chronic
diseases such as atherosclerosis, diabetes mellitus, kidney injury, and stroke.
We investigated the role of total salvianolic acids (TSA), total tanshinones (TTSN),
and their combination (TSA/TTSN) on the activated vascular endothelial cell and
its inhibitory effect on signal transduction and cytokines regulation. In the extracellular
medium of the injury model of human umbilical vein endothelial cells (HUVECs) induced
by thrombin, the human IL-6, VCAM-1, and ICAM-1 were significantly elevated (p ˂
0.05). However, suppression in the TSA, TTSN, and TSA/TTSN (100 µg/L)-treated groups
(p > 0.05) were notable. TSA alone but not TTSN and TSA/TTSN combination, inhibited
the expression of P-selectin (p < 0.05) and E-selectin (p < 0.01) respectively,
in VECs. Western blot analysis showed JAK and STAT expression in VECs however, the
protein expression was modest in the Salvia miltiorrhiza-treated groups, indicating
the potential of TSA/TTSN in the inflammatory pathways of IL-6, JAK, and STAT signal
transduction in endothelial cells (ECs). This study has made novel observations
regarding the components of Salvia miltiorrhiza regulatory effect on cytokines in
Vascular Biology.
References:
[1] Cahill, P.A., and E.M. Redmond, Vascular endothelium - Gatekeeper of vessel health. Atherosclerosis, 2016. 248: p. 97-109.
[2] Al-Soudi, A., M.H. Kaaij, and S.W. Tas, Endothelial
cells: from innocent bystanders to active participants in immune responses.
Autoimmunity Reviews, 2017. 16(9): p. 951-962.
[3] Pi, X., L. Xie, and C. Patterson, Emerging Roles of
Vascular Endothelium in Metabolic Homeostasis. Circulation Research, 2018.
123(4): p. 477-494.
[4] Mundi, S., et al., Endothelial permeability, LDL
deposition, and cardiovascular risk factors—a review. Cardiovascular Research,
2017. 114(1): p. 35-52.
[5] Singh, C., C.G. Pfeifer, and W.A. Jefferies,
Pathogenic Angiogenic Mechanisms in Alzheimer’s. Physiologic and Pathologic
Angiogenesis: Signaling Mechanisms and Targeted Therapy, 2017: p. 93.
[6] Wolf, D. and K. Ley, Immunity and Inflammation in
Atherosclerosis. Circulation Research, 2019. 124(2): p. 315-327.
[7] Nasonov, E.L. and T.V. Popkova, Atherosclerosis:
perspectives of anti-inflammatory therapy. Ter Arkh, 2018. 90(5): p. 4-12.
[8] Grandl, G. and C. Wolfrum, Hemostasis, endothelial
stress, inflammation, and the metabolic syndrome. Semin Immunopathol, 2018.
40(2): p. 215-224.
[9] Domingueti, C.P., et al., Diabetes mellitus: The
linkage between oxidative stress, inflammation, hypercoagulability, and
vascular complications. Journal of Diabetes and its Complications, 2016. 30(4):
p. 738-745.
[10] Tacey, A. et al., The effect of an atherogenic diet
and acute hyperglycaemia on endothelial function in rabbits is artery specific.
Nutrients, 2020. 12(7): p. 2108.
[11] Cade, W.T., Diabetes-related microvascular and
macrovascular diseases in the physical therapy setting. Phys Ther, 2008.
88(11): p. 1322-35.
[12] Palta, S., R. Saroa, and A. Palta, Overview of the
coagulation system. Indian Journal of Anaesthesia, 2014. 58(5): p.
515-523.
[13] Göbel, K., et al., The Coagulation Factors
Fibrinogen, Thrombin, and Factor XII in Inflammatory Disorders-A Systematic
Review. Frontiers in immunology, 2018. 9: p. 1731-1731.
[14] Petrey, A.C., and C.A. de la Motte, Thrombin
Cleavage of Inter-α-inhibitor Heavy Chain 1 Regulates Leukocyte Binding to an
Inflammatory Hyaluronan Matrix. The Journal of Biological Chemistry,
2016. 291(47): p. 24324-24334.
[15] Wadowski, P.P., et al., Protease-activated
receptor-mediated platelet aggregation in acute coronary syndrome patients on
potent P2Y(12) inhibitors. Research and practice in thrombosis and haemostasis,
2019. 3(3): p. 383-390.
[16] Orgah, J.O., et al., Pharmacological potential of
the combination of Salvia miltiorrhiza (Danshen) and Carthamus tinctorius
(Honghua) for diabetes mellitus and its cardiovascular complications.
Pharmacological Research, 2020. 153: p. 104654.
[17] Xu, J., et al., Ethnopharmacology, phytochemistry,
and pharmacology of Chinese Salvia species: A review. J Ethnopharmacol, 2018. 225:
p. 18-30.
[18] Qi, Y., et al., Identification of a Quality Marker
(Q-Marker) of Danhong Injection by the Zebrafish Thrombosis Model. Molecules,
2017. 22(9).
[19] Li, G., et al., Comparison of the chromatographic
fingerprint, multicomponent quantitation and antioxidant activity of Salvia
miltiorrhiza Bge. between sweating and non-sweating. 2018. 32(6): p. e4203.
[20] Luo, H., et al., Quality evaluation of Salvia
miltiorrhiza Bge. by ultra-high performance liquid chromatography with
photodiode array detection and chemical fingerprinting coupled with chemometric
analysis. J Sep Sci, 2015. 38(9): p. 1544-51.
[21] Maione, F. et al., Tanshinone IIA, a major component
of Salvia milthorriza Bunge, inhibits platelet activation via the Erk-2
signaling pathway. J Ethnopharmacol, 2014. 155(2): p. 1236-42.
[22] Chen, Z. et al., Tanshinone IIA Exerts
Anti-Inflammatory and Immune-Regulating Effects on Vulnerable Atherosclerotic
Plaque Partially via the TLR4/MyD88/NF-kappaB Signal Pathway. Front Pharmacol,
2019. 10: p. 850.
[23] Onat, D., et al., Human vascular endothelial cells:
a model system for studying vascular inflammation in diabetes and
atherosclerosis. Current diabetes reports, 2011. 11(3): p. 193-202.
[24] Biondi-Zoccai, G.G., et al., Atherothrombosis,
inflammation, and diabetes. Journal of the American College of Cardiology,
2003. 41(7): p. 1071-1077.
[25] Bogatcheva, N., J. Garcia, and A. Verin, Molecular
mechanisms of thrombin-induced endothelial cell permeability. Biochemistry
(Moscow), 2002. 67(1): p. 75-84.
[26] Wagner, D.D. and P.C. Burger, Platelets in
inflammation and thrombosis. Arteriosclerosis, thrombosis, and vascular
biology, 2003. 23(12): p. 2131-2137.
[27] Gawaz, M., H. Langer, and A.E. May, Platelets in
inflammation and atherogenesis. Journal of Clinical Investigation, 2005.
115(12): p. 3378-3384.
[28] Somers, W.S. et al., Insights into the Molecular
Basis of Leukocyte Tethering and Rolling Revealed by Structures of P- and
E-Selectin Bound to SLeX and PSGL-1. Cell, 2000. 103(3): p. 467-479.
[29] Kontzias, A., et al., Jakinibs: a new class of
kinase inhibitors in cancer and autoimmune disease. Current Opinion in
Pharmacology, 2012. 12(4): p. 464-470.
[30] Bai, K.J., et al., Thrombin-induced CCN2 expression
in human lung fibroblasts requires the c-Src/JAK2/STAT3 pathway. J Leukoc Biol,
2013. 93(1): p. 101-12.
[31] Shi, G.P., Immunomodulation of Vascular Diseases:
Atherosclerosis and Autoimmunity. European journal of vascular and endovascular
surgery: the official journal of the European Society µ for Vascular Surgery,
2010. 39(4): p. 485-494.
[32] Akash, M.S.H., K. Rehman, and S. Chen, Role of
inflammatory mechanisms in the pathogenesis of type 2 diabetes mellitus.
Journal of cellular biochemistry, 2013. 114(3): p. 525-531.
[33] Tabit, C.E., et al., Endothelial dysfunction in
diabetes mellitus: molecular mechanisms and clinical implications. Reviews in
Endocrine and Metabolic Disorders, 2010. 11(1): p. 61-74.
[34] Manea, S.-A., A. Manea, and C. Heltianu, Inhibition
of JAK/STAT signaling pathway prevents a high-glucose-induced increase in
endothelin-1 synthesis in human endothelial cells. Cell and Tissue Research,
2010. 340(1): p. 71-79.
[35] Pigott, R. et al., Soluble forms of E-selectin,
ICAM-1, and VCAM-1 are present in the supernatants of cytokine activated
cultured endothelial cells. Biochemical and Biophysical Research
Communications, 1992. 187(2): p. 584-589.
[36] Jude, E.B., et al., Circulating cellular adhesion
molecules ICAM-1, VCAM-1, P- and E-selectin in the prediction of cardiovascular
disease in diabetes mellitus. European Journal of Internal Medicine, 2002.
13(3): p. 185-189.
[37] Xie, Z., et al., Dan-Qi prescription ameliorates
insulin resistance through overall corrective regulation of glucose and fat
metabolism. Journal of ethnopharmacology, 2015. 172: p. 70-79.
[38] Chen, X., et al., Tanshinone II Aattenuates renal
damage in STZ-induced diabetic rats via inhibiting oxidative stress and
inflammation. Oncotarget, 2017. 8(19): p. 31915.

